China's NMPA has received Henlius' NDA for HLX11, a biosimilar of Pertuzumab
Shanghai Henlius Biotech, Inc. (2696.HK) has reported that the National Medical Products Administration (NMPA) has accepted the new drug application (NDA) for its proprietary pertuzumab biosimilar HLX11. This biosimilar is intended for use alongside trastuzumab and chemotherapy as an adjuvant therapy for patients with HER2-positive early breast cancer who are at a significant risk of recurrence. It is also indicated for patients with HER2-positive metastatic or unresectable locally recurrent breast cancer, who have not previously undergone anti-HER2 therapy or chemotherapy for their metastatic condition, when used in combination with trastuzumab and docetaxel.
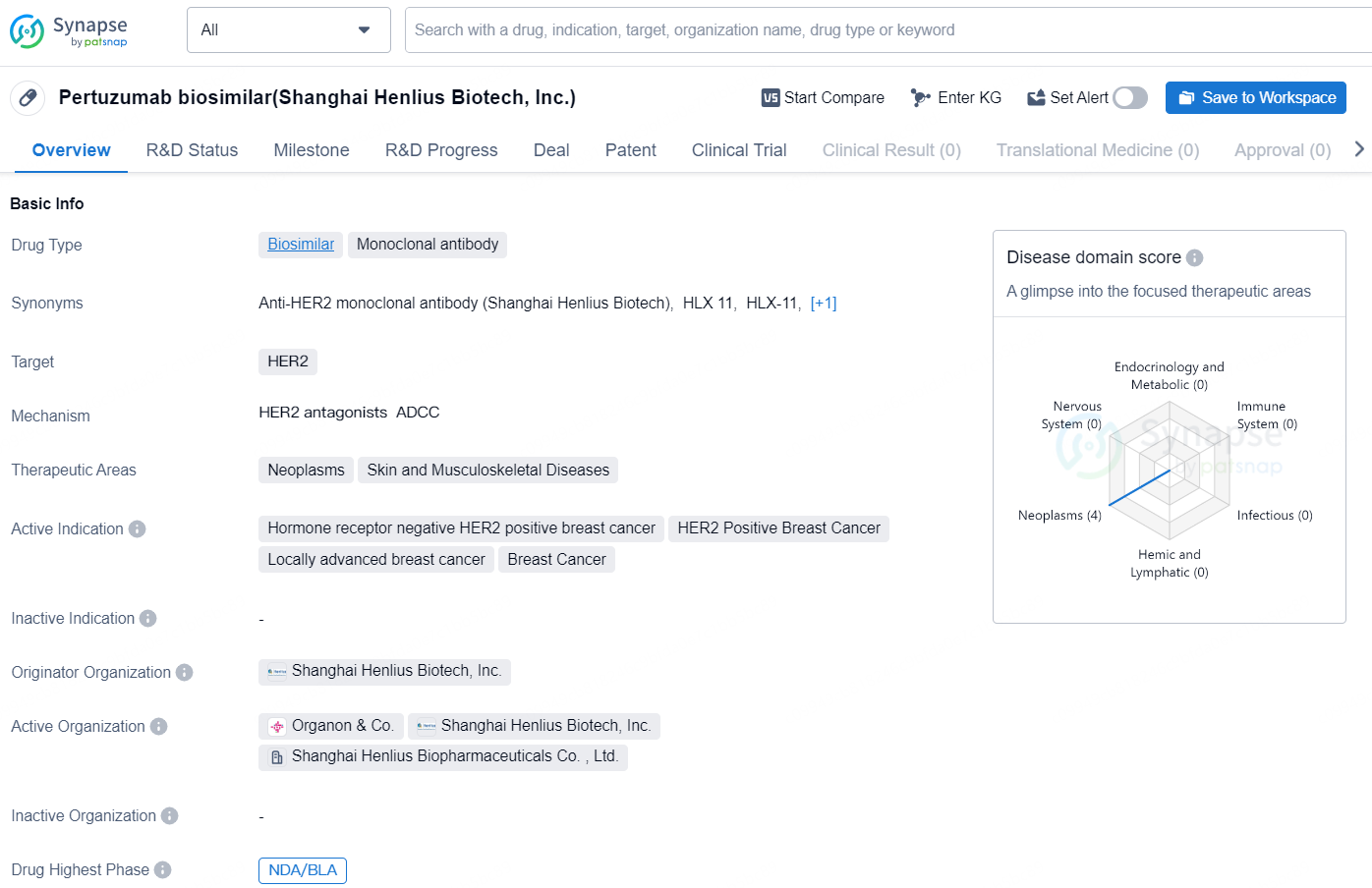
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
HLX11 is a biosimilar of pertuzumab, developed by Henlius in accordance with the applicable regulations and guidelines pertaining to biosimilars in China, the United States, and the European Union. The submission for approval was supported by extensive data derived from a series of comparative studies between HLX11 and the reference pertuzumab. This included analytical similarity assessments, phase 1 clinical trials, and international multicenter phase 3 clinical investigations. The accumulated evidence indicates that HLX11 closely resembles the reference pertuzumab with regard to quality, safety, and efficacy.
Breast cancer ranks as the second most prevalent cancer globally and is the most frequently diagnosed cancer among women. According to GLOBOCAN, there were 2.3 million new breast cancer cases reported worldwide in 2022, with 357,200 of these occurring in China. Among these cases, HER2-positive breast cancer represents approximately 20-25% of all breast cancers. Such tumor cells are distinguished by their aggressive nature, high malignancy, and rapid progression. Pertuzumab targets subdomain II of HER2's extracellular domain, effectively blocking the heterodimerization of HER2 with other HER family receptors, such as EGFR, HER3, and HER4. This interference halts signal transduction through associated pathways, ultimately leading to tumor cell growth cessation and apoptosis.
Additionally, pertuzumab enhances the antitumor activity of immune cells via antibody-dependent cellular cytotoxicity. Clinical trials have demonstrated that the combination of pertuzumab and trastuzumab shows strong synergistic effects, significantly enhancing clinical outcomes and improving patient prognosis. This dual-target therapy, incorporating both pertuzumab and trastuzumab, has become a foundational treatment approach for HER2-positive breast cancer, endorsed by multiple clinical guidelines, including those from the National Comprehensive Cancer Network (NCCN), the Chinese Society of Clinical Oncology (CSCO), and the China Anti-Cancer Association (CACA).
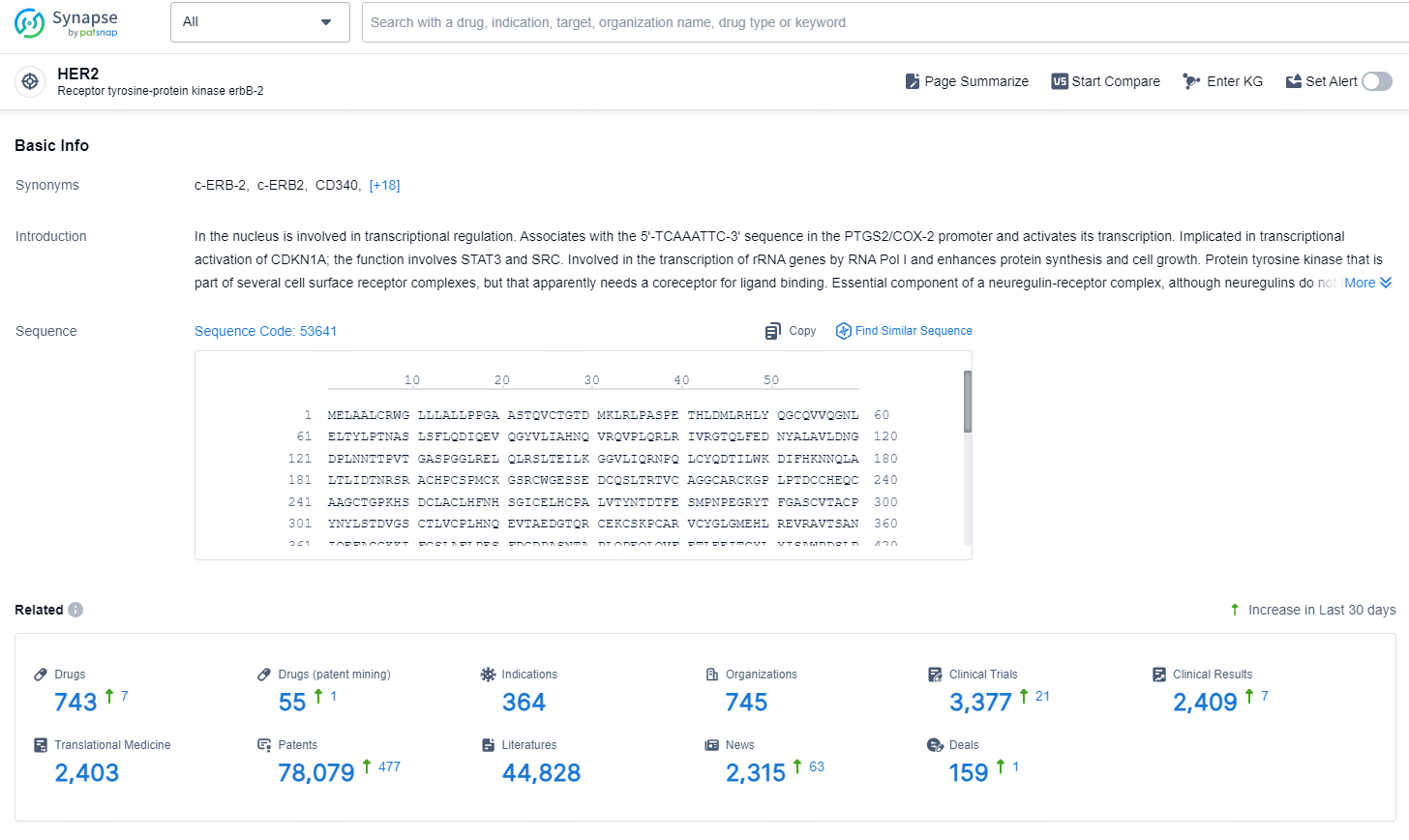
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 10, 2024, there are 743 investigational drugs for the HER2 target, including 364 indications, 745 R&D institutions involved, with related clinical trials reaching 3377, and as many as 78079 patents.
Pertuzumab biosimilar is a monoclonal antibody drug type developed by Shanghai Henlius Biotech, Inc. It is a biosimilar drug that targets HER2, and is used in the treatment of neoplasms, skin and musculoskeletal diseases. The drug is indicated for hormone receptor negative HER2 positive breast cancer, HER2 Positive Breast Cancer, Locally advanced breast cancer, and Breast Cancer.