Circle Pharma Launches Phase 1 Trial of CID-078, Oral Inhibitor for Advanced Solid Tumors
Circle Pharma, Inc., a biopharmaceutical firm in the clinical stage focusing on the discovery and development of next-generation macrocycle treatments, reported that dosing has commenced for the first group of patients in their phase 1 trial of CID-078. This trial assesses CID-078, an innovative oral cyclin A/B RxL inhibitor, in patients with advanced solid tumors. The study includes those with high E2F transcription factor activity, such as small cell lung cancer, triple negative breast cancer, and ER+ HER-2- breast cancer, particularly after treatment with CDK 4/6 inhibitors.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
David J. Earp, JD, Ph.D., CEO of Circle Pharma, expressed his excitement over the recent approval of the IND for CID-078 following the 30-day regulatory review, allowing the compound to advance to human trials in collaboration with premier cancer centers. This progression will enable researchers to gather clinical proof-of-concept data that can determine CID-078’s potential in addressing critical unmet medical needs, while also providing validation for Circle Pharma's proprietary MXMO™ macrocycle platform, which targets challenging cancer-related proteins and other severe diseases.
CID-078 is engineered to specifically block crucial protein-to-protein interactions involving cyclins A and B—both linked to the growth and endurance of cancer cells. The research relies on foundational work by William G. Kaelin Jr., MD, a Nobel Laureate and Chair of Circle Pharma's Scientific Advisory Board, who demonstrated synthetic lethality by disrupting these cyclins in contexts of unregulated cell cycle control and increased E2F activity.
Dr. Geoffrey Shapiro, MD, Ph.D., senior vice president, Development Therapeutics at Dana-Farber Cancer Institute and professor of medicine at Harvard University, noted, “Interfering with the ability of E2F-driven cancer cells to deactivate E2F at the right point in the cell cycle has been proven to selectively cause apoptosis. Previously, there wasn’t a specific therapeutic agent to harness this insight, so I am very excited to see Circle Pharma’s cyclin A/B RxL inhibitor entering clinical trials.”
Patient recruitment is underway at The START Center for Cancer Research, Midwest, Grand Rapids, MI, with additional centers open at The START Center for Cancer Research, West Valley City, UT, and NEXT Oncology in San Antonio, TX. More clinical sites are expected to launch soon.
Circle Pharma has initiated a Phase 1 clinical trial (NCT06577987), an open-label, multi-center dose-escalation and expansion study, aiming to enroll up to 100 patients. The trial will assess the safety, pharmacokinetics, pharmacodynamics, and preliminary anti-tumor efficacy of CID-078 in solid tumors. Circle Pharma aims to release initial safety and anti-tumor findings from this Phase 1 trial in 2025.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
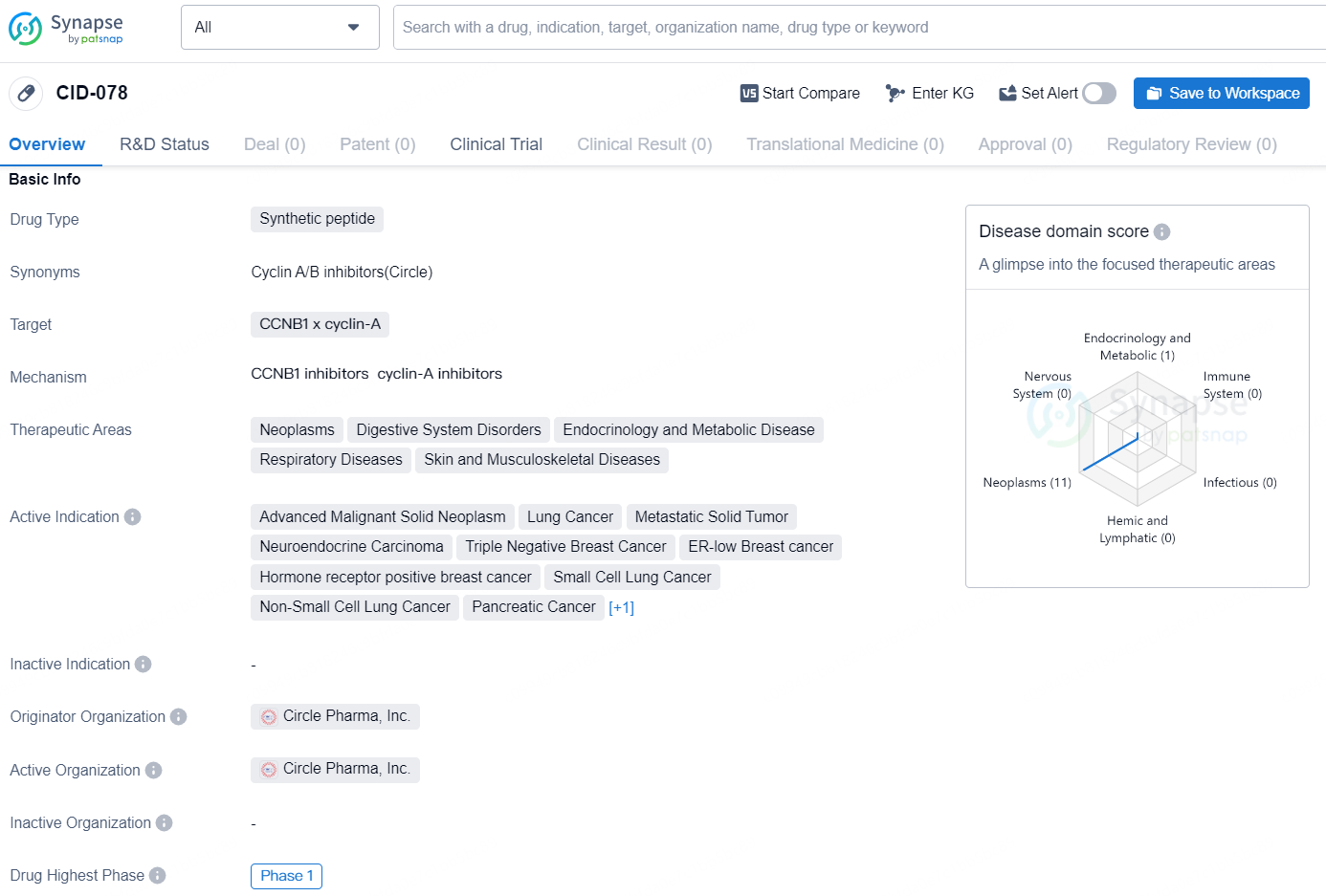
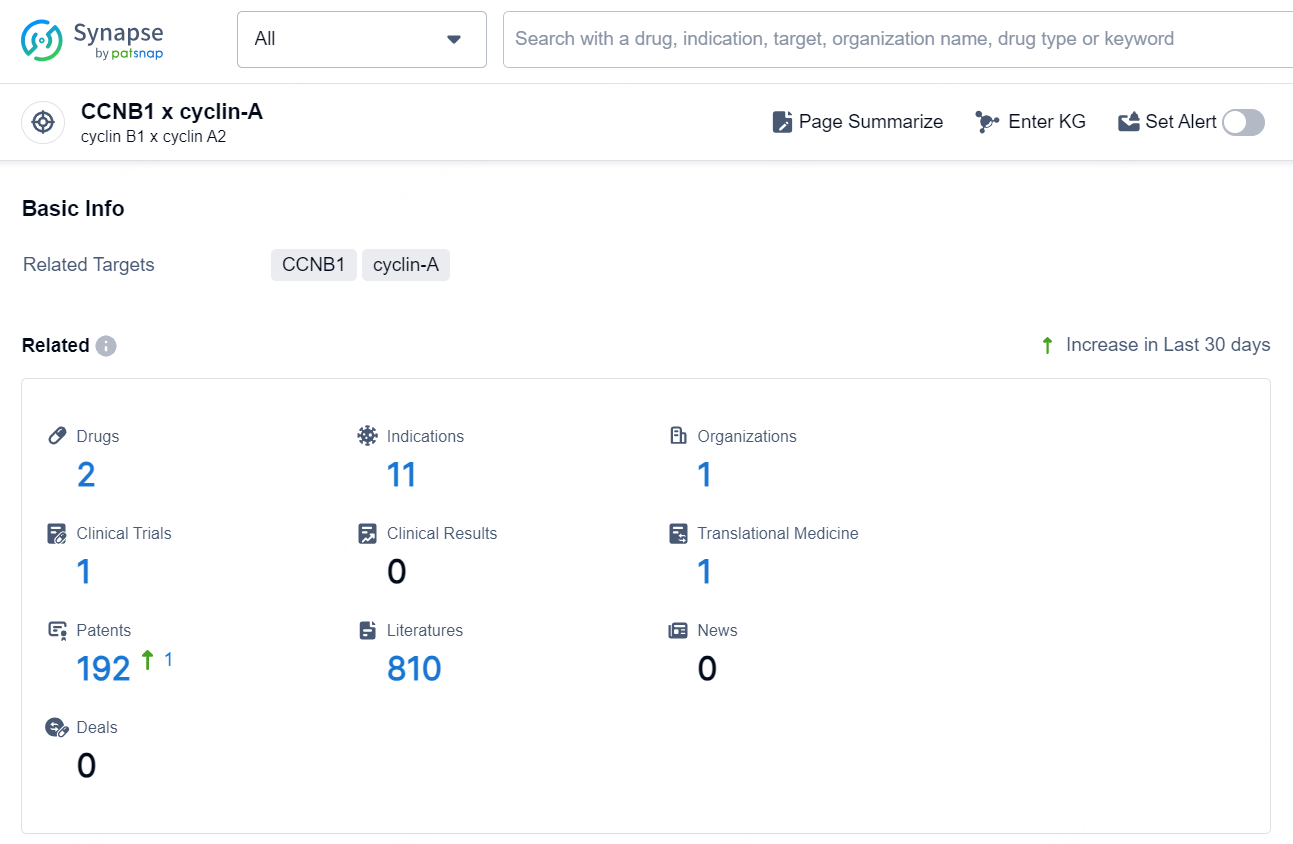
According to the data provided by the Synapse Database, As of October 16, 2024, there are 2 investigational drugs for the CCNB1 x cyclin-A target, including 11 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 192 patents.
The drug [CID-078] is a synthetic peptide that targets the CCNB1 x cyclin-A proteins. It is indicated for the treatment of a wide range of therapeutic areas including Neoplasms, Digestive System Disorders, Endocrinology and Metabolic Disease, Respiratory Diseases, and Skin and Musculoskeletal Diseases. The drug is specifically intended for the treatment of various types of advanced malignant solid neoplasms, lung cancer, metastatic solid tumors, neuroendocrine carcinoma, triple negative breast cancer, ER-low breast cancer, hormone receptor positive breast cancer, small cell lung cancer, non-small cell lung cancer, pancreatic cancer, and stomach cancer.