Clinical trials starts for DS-3939, aimed at treating a variety of complex Advanced Solid Cancers
Daiichi Sankyo has declared that the first patient has been dosed in a first-in-human Phase 1/2 examination evaluating DS-3939. This study focuses on individuals suffering from various kinds of severe solid tumors which includes non-small cell lung, breast, urothelial, ovarian, biliary tract, and pancreatic cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
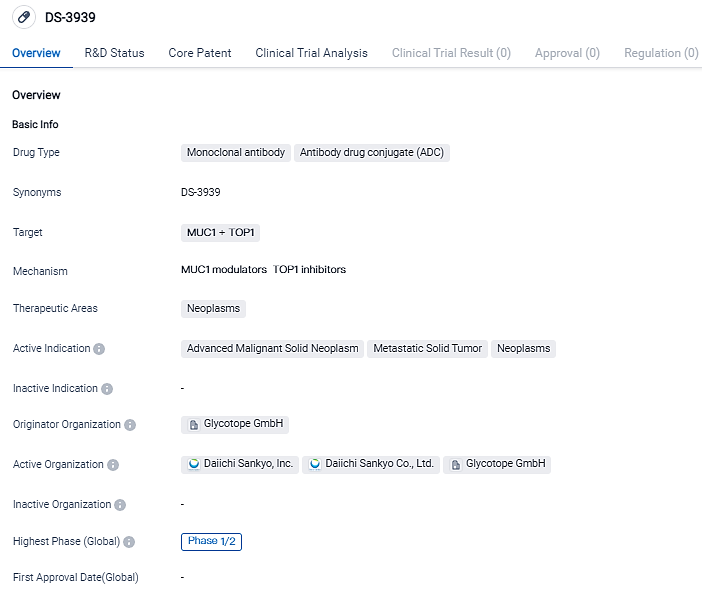
DS-3939 is a specially designed potential first-in-class tumor-associated mucin-1 (TA-MUC1) specific antibody drug compound, formulated using the exclusive DXd ADC technology from Daiichi Sankyo. TA-MUC1 is a cancer-specific transmembrane glycoprotein that exhibits overexpression in the majority of human epithelial cancers, thereby posing as an ideal candidate for cancer treatment. At present, no therapies targeting TA-MUC1 have received approval for any sort of cancer.
"Through DS-3939, we are leveraging our distinctive DXd antibody drug conjugate methodology and pairing it with a TA-MUC1 antibody to examine this innovative treatment strategy in patients who are dealing with various kinds of advanced cancer," stated Mark Rutstein, MD, Global Head of Oncology Clinical Development at Daiichi Sankyo.
The phase 1/2 trial, which is split into two sections, involves multiple centers and adopts an open-label, first-in-human approach. This will evaluate the effectiveness and safety of DS-3939 in patients suffering from locally advanced, metastatic or inoperable solid tumors not susceptible to standard therapy for each respective tumor type.
The first section of the trial focuses on the safety and tolerability of incremental doses of DS-3939 to establish the maximum tolerated dose or the advised dosages for expansion in patients with locally advanced, metastatic, or inoperable solid tumors.
The second section comprises several expansion cohorts to further examine the effectiveness and safety of DS-3939. This trial will incorporate safety endpoints, which includes dose-limiting toxicities and adverse events, and efficacy endpoints comprising overall response rate, disease control rate, response duration, response time, progression-less survival, and overall survival. It will also probe pharmacokinetic and biomarker endpoints.
DS-3939, a potential first-in-class TA-MUC1 directed ADC, is currently under investigation. Developed using Daiichi Sankyo’s proprietary DXd ADC technology, DS-3939 includes a humanized anti-TA-MUC1 antibody licensed from Glycotope GmbH, attached to a series of topoisomerase I inhibitor payloads via tetrapeptide-based breakable linkers.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
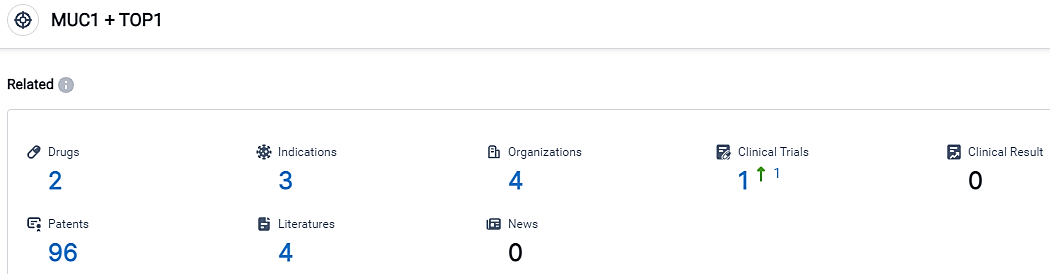
According to the data provided by the Synapse Database, As of September 12, 2023, there are 2 investigational drugs for the MUC1 and TOP1 target, including 3 applicable indications,4 R&D institutions involved, with related clinical trials reaching 1,and as many as 96 patents.
DS-3939 is a monoclonal antibody and antibody drug conjugate that targets MUC1 and TOP1 proteins. It is being developed by Glycotope GmbH for the treatment of neoplasms, specifically advanced malignant solid neoplasms and metastatic solid tumors. The drug is currently in Phase 1/2 of clinical development, indicating its potential as a future treatment option. Further research and clinical trials are needed to determine the drug's effectiveness and safety.