Contemporary Competitive Scenario: Analyzing Roche's Emergent Product, Trontinemab
A year prior, Roche publicly announced at an Alzheimer's disease conference held in San Francisco that its prospective gantenerumab - an antibody developed against amyloid protein, displayed no significant improvement in cognitive decline as per a pivotal study involving 2,000 patients. The experiment's results, showing a lower-than-anticipated removal of the amyloid protein, led Roche to suspend additional research on this medication.
However, Roche remained undeterred in pursuing groundbreaking research and development within this disease domain. At this year's Clinical Trials on Alzheimer's Disease (CTAD) conference, Roche gathered initial data on a refined antibody deriving from gantenerumab. This transformed version, however, was designed to permeate the protective blood-brain barrier with more ease than its predecessor.
That new drug, called trontinemab, displays an eight-fold increase in concentration within the cerebrospinal fluid compared to gantenerumab. At the conference, Roche announced that after 28 weeks, 75% of the patients who were administered the highest dosage among three different dosages exhibited amyloid protein levels beneath the average detection level.
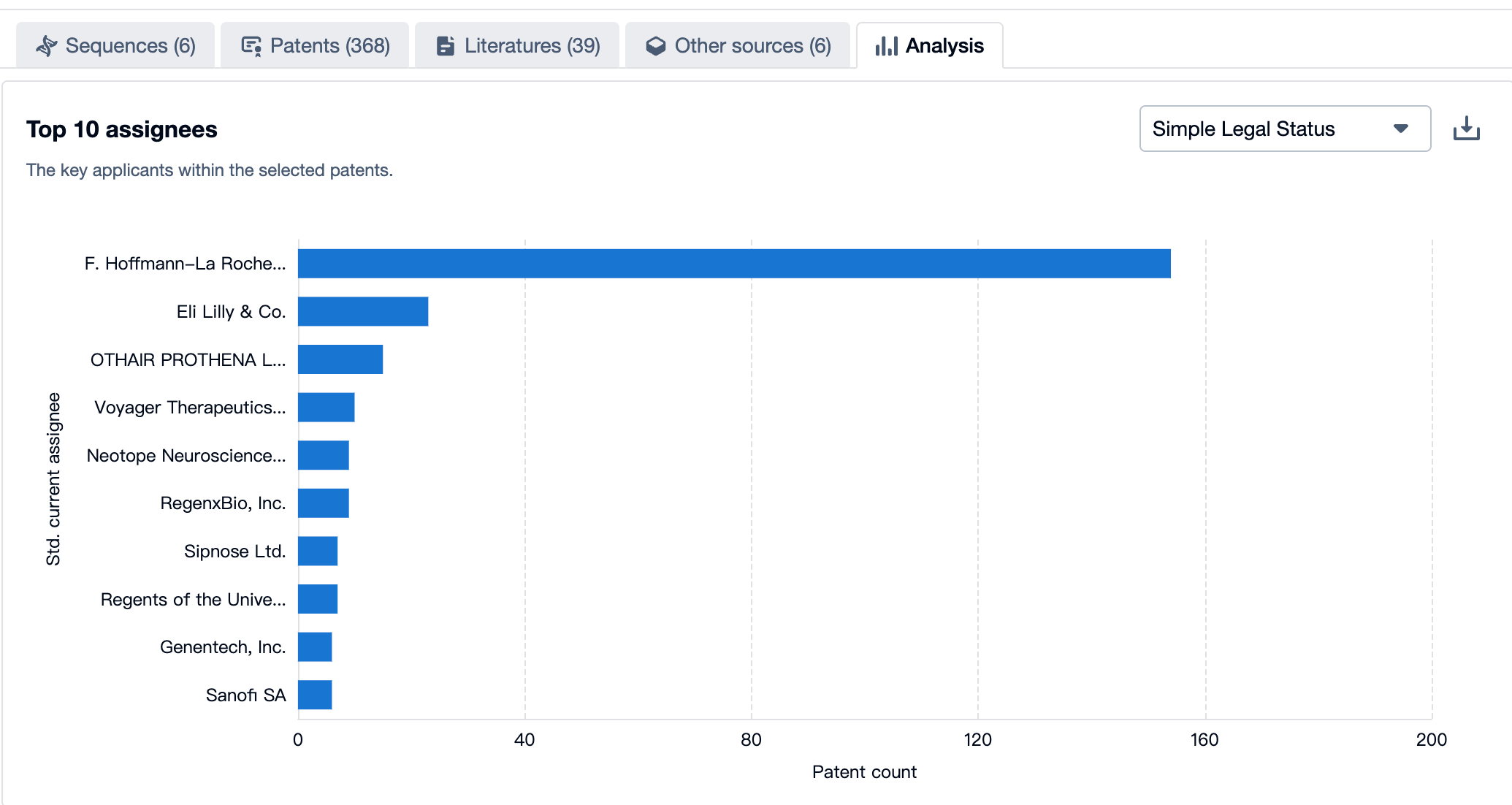
A Panoramic View of Trontinemab’s Top 10 Patent Assignees
Source, Patsnap Bio
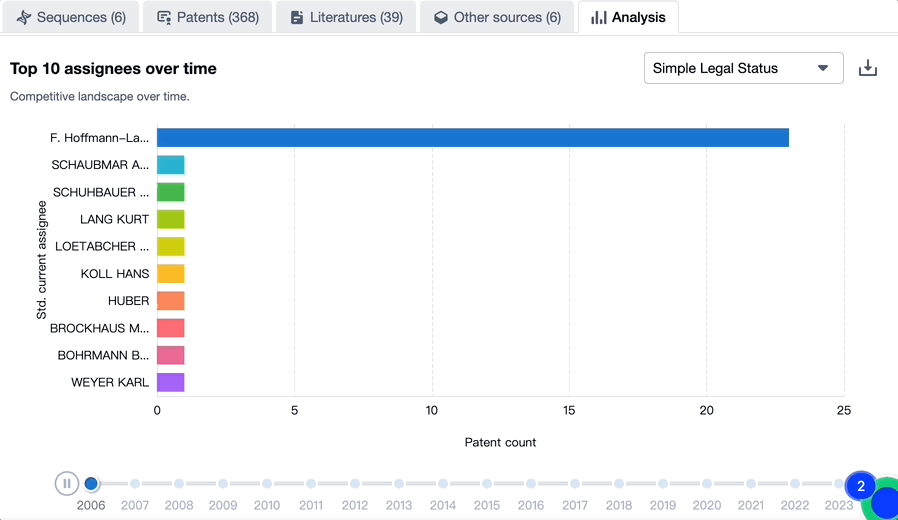
Evolution of the Layout of Top 10 Patent Assignees over the years, the Competitive landscape over time
Exploring the changes occurring within the application structure amongst Trontinemab's top 10 patent assignees over time delivers insights into the competitive landscape's evolution.
Source, Patsnap Bio
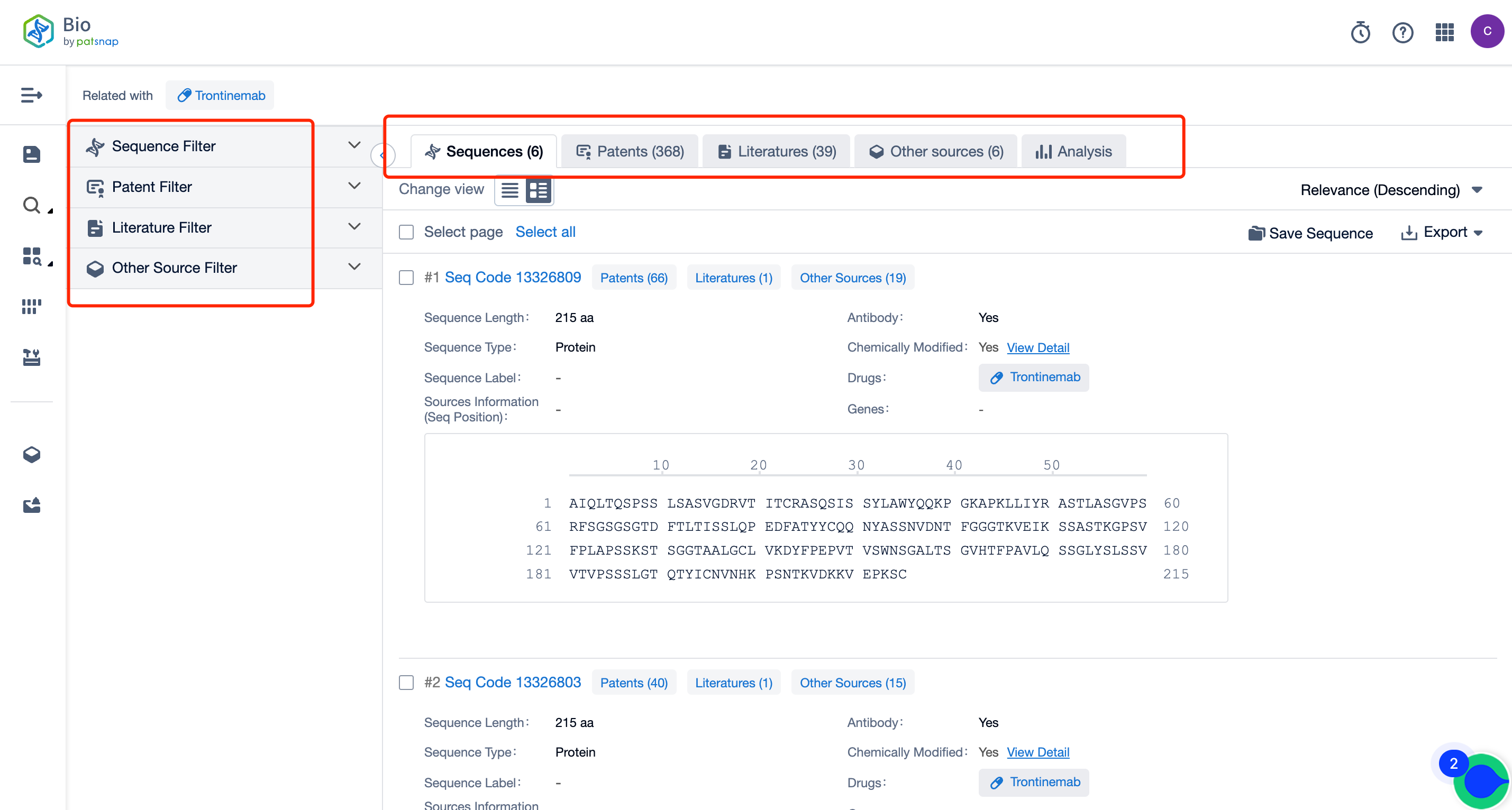
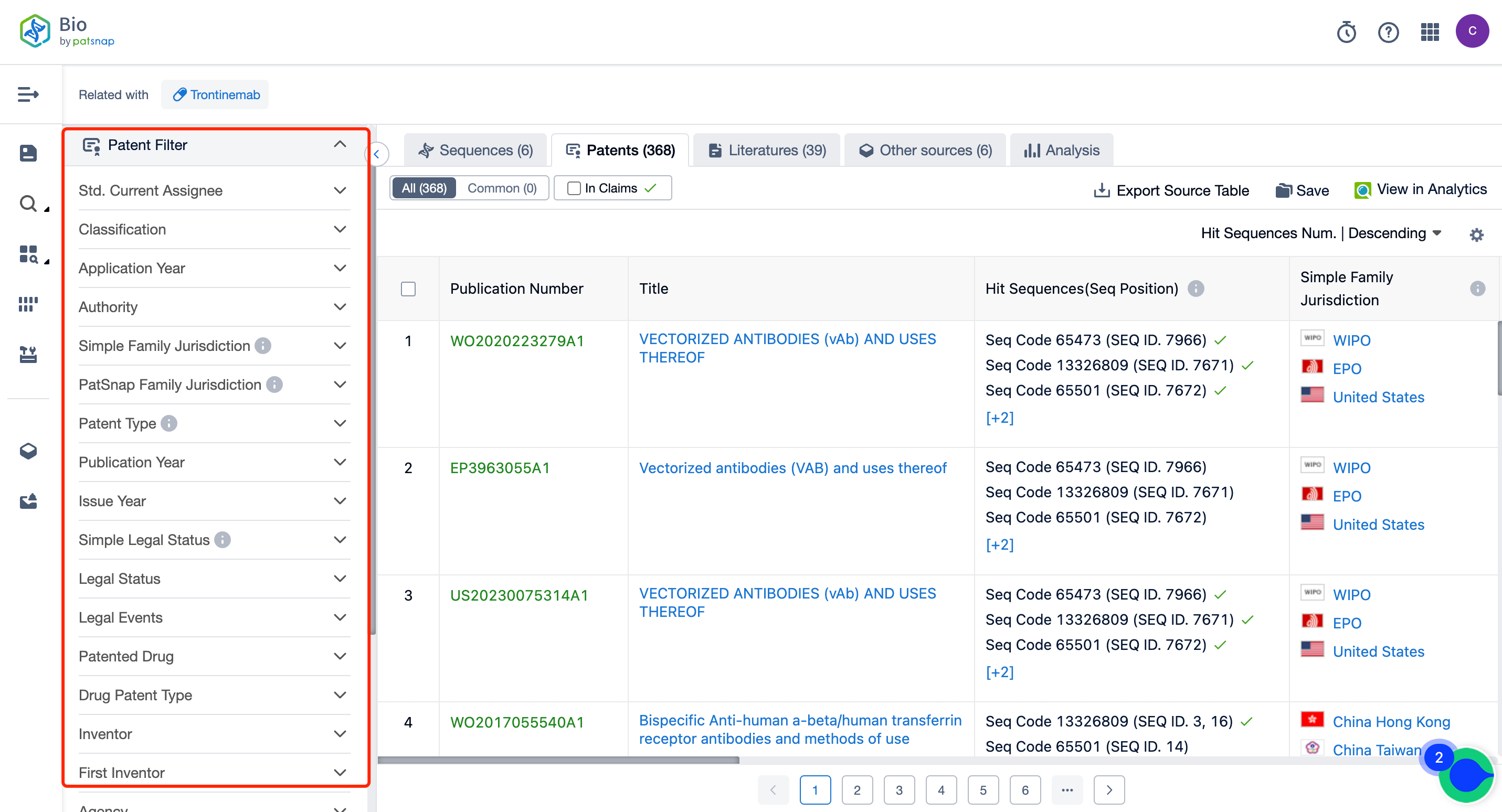
So, How Can You Access Comprehensive Data on Trontinemab Sequence Patents at No Cost?
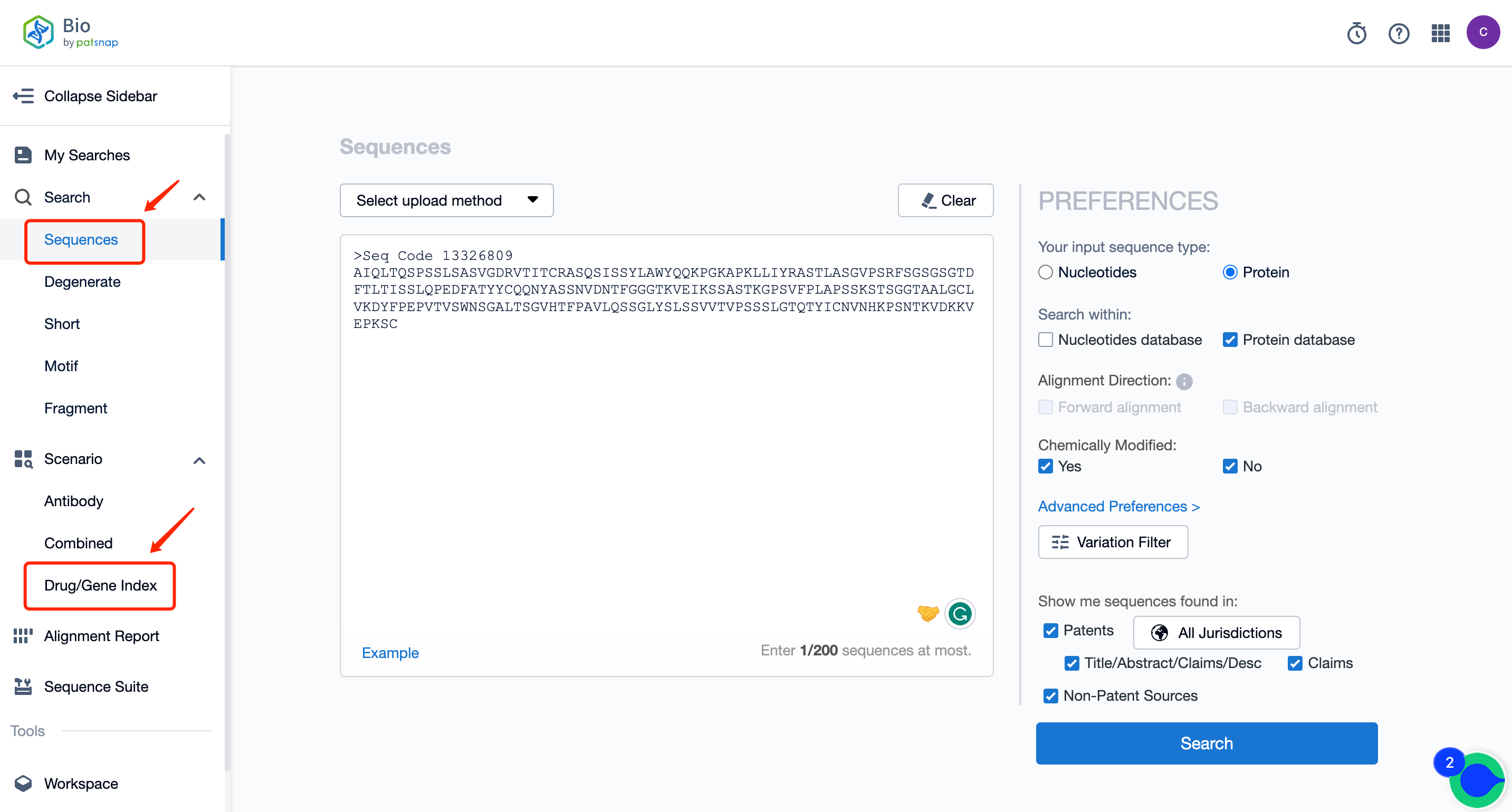
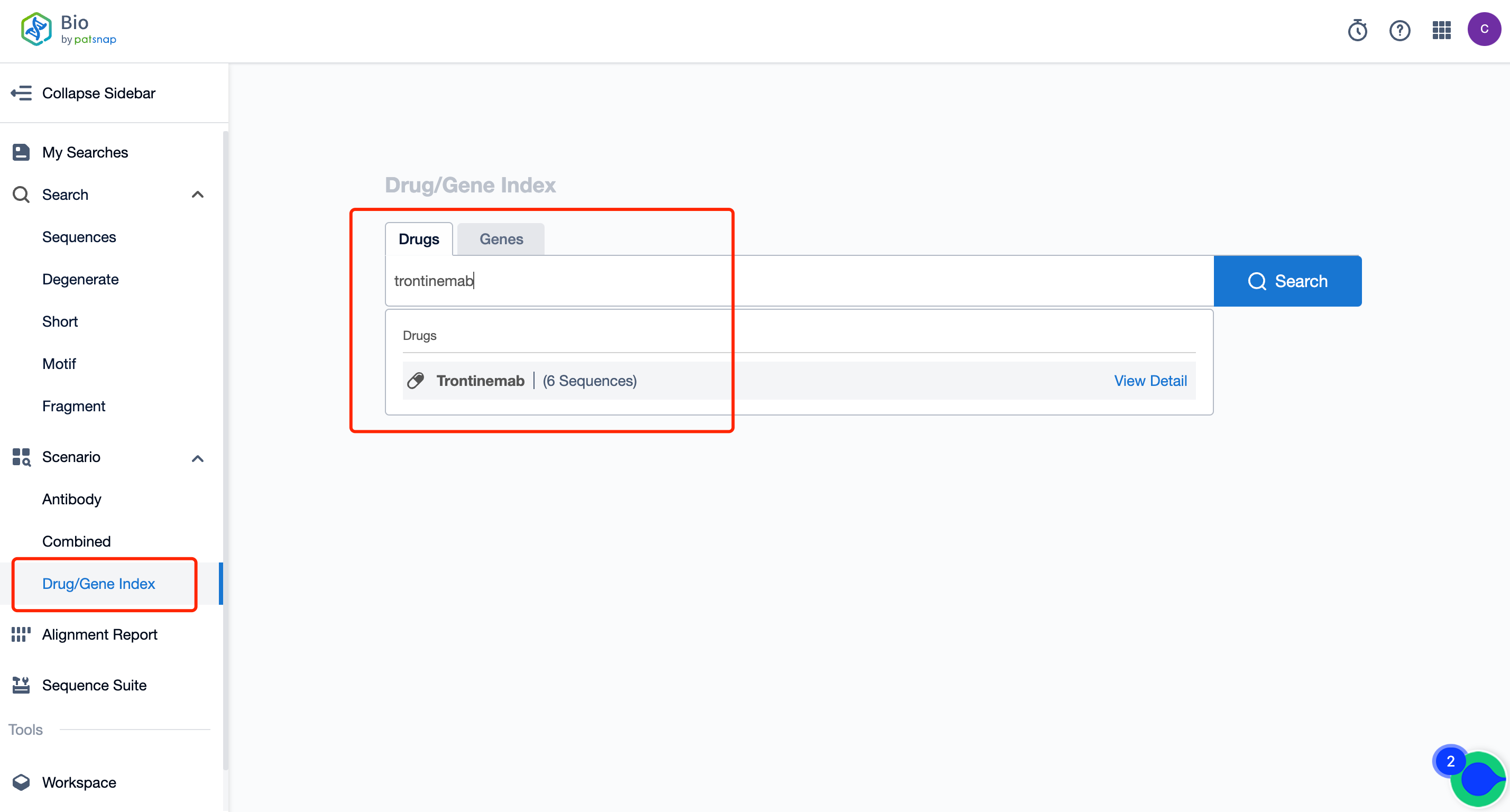
Firstly, establish a free account with Patsnap Bio Sequence Database. Proceed to the homepage's "standard search," and enter the Trontinemab sequence or directly input the drug name, Trontinemab, in the "Drug/Gene index.” This single action will unravel extensive details of Trontinemab's sequence, patent, literature, data from diversified sources, and a visually competitive landscape of patents.
The left panel's search result details page is equipped with an exhaustive range of filters, enabling you to pinpoint specific data accurately, thereby boosting your search experience and overall efficiency. Clicking on each data point will unfold a rich and detailed data set and a host of advantageous and practical tools to support your research process.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio-patsnap-com.libproxy1.nus.edu.sg. Act now to expedite your sequence search tasks.