CRISPR Technology: Revolutionizing Genetic Medicine and Driving Market Growth
According to a report by Yole Développement, the CRISPR technology market is projected to reach $5 billion by 2023, marking its rapid global development and widespread application. By 2027, the global CRISPR technology market is expected to grow to $3.94 billion, with a compound annual growth rate (CAGR) of 24.2%. This forecast underscores the sustained growth of CRISPR technology across various fields, particularly in therapeutic applications. Key therapeutic uses of CRISPR include gene editing, gene therapy, and immunotherapy, which hold promise for offering new treatment solutions for numerous genetic disorders and cancers.
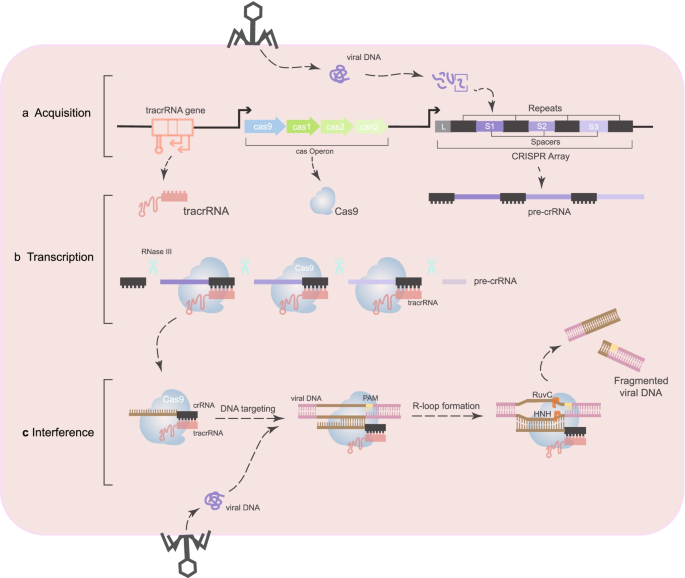
Mechanism of CRISPR-Cas9
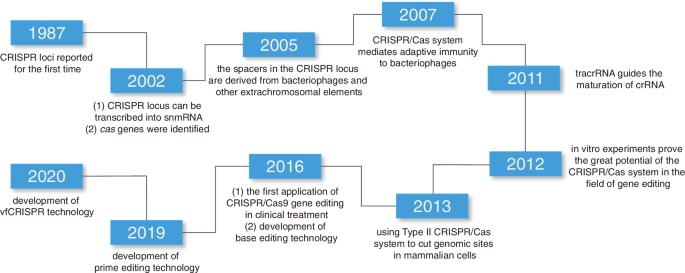
The development of CRISPR-Cas9 technology can be traced back to 1987, when Japanese scientists first identified CRISPR sequences in Escherichia coli, though their significance was not realized at the time. In 2005, researchers discovered that CRISPR sequences matched viral DNA infecting bacteria, revealing the relationship between CRISPR and bacterial defense against viruses. By 2007, researchers at Danisco demonstrated that the CRISPR system could function as an adaptive immune mechanism in bacteria, protecting against foreign genetic material.
In 2012, Jennifer Doudna, Emmanuelle Charpentier, and their team unveiled the functional mechanism of the CRISPR-Cas9 system. They discovered that the Cas9 protein, guided by RNA molecules, could precisely identify and cleave specific DNA sequences, highlighting its immense potential for precise gene editing. This breakthrough rapidly made CRISPR-Cas9 a prominent tool in scientific research, finding applications in gene function studies, genetic disease treatments, and more.
In the CRISPR-Cas system, CRISPR loci are initially transcribed into a long precursor RNA (pre-crRNA). This process occurs within bacterial or archaeal cells in response to the invasion of viruses or other foreign genetic material. CRISPR loci consist of multiple repeat sequences and spacer sequences, the latter of which are derived from fragments of previously encountered viral genes. Typically, CRISPR loci include a leader region rich in AT bases, which acts as a promoter. Following the leader region, alternating repeat and spacer sequences form a pattern of "repeat-spacer-repeat," transcribed under the leader region's regulation into pre-crRNA containing multiple repeats and spacers. Each spacer corresponds to an integrated viral gene fragment.
Pre-crRNA requires processing to form mature crRNA, a process aided by trans-activating crRNA (tracrRNA). TracrRNA is a longer RNA molecule that contains a domain binding to Cas proteins and a sequence complementary to the repeat regions of pre-crRNA. Through complementary pairing, tracrRNA binds to the repeat regions of pre-crRNA to form a double-stranded RNA structure. This structure is cleaved by endogenous enzymes like RNase III, producing multiple mature crRNA molecules. Each crRNA includes a spacer sequence complementary to a previously integrated viral gene fragment. Mature crRNA, approximately 20 nucleotides long, specifically recognizes target DNA sequences.
The mature crRNA is produced from pre-crRNA and includes a spacer sequence complementary to the integrated viral gene fragment. TracrRNA facilitates the complementary pairing of its sequence to the repeat regions of pre-crRNA, forming a double-stranded RNA structure. Enzymes such as RNase III cleave this structure to produce mature crRNA, which includes a spacer sequence complementary to the target DNA sequence. Mature crRNA pairs with tracrRNA to form a double-stranded RNA complex, structurally resembling a "Y" shape. The spacer sequence of crRNA remains single-stranded, while the remainder forms a double strand with tracrRNA. This double-stranded RNA complex stabilizes the crRNA and provides the necessary structure for subsequent binding to the Cas9 protein.
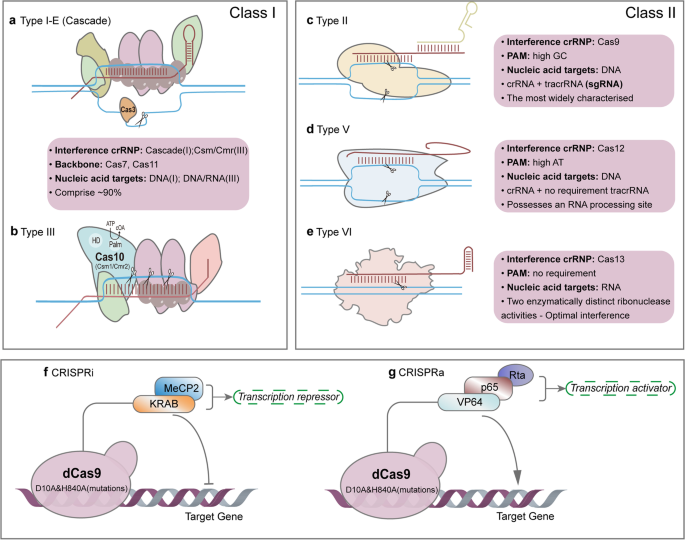
Cas9 protein is a nuclease capable of cleaving DNA at specific locations. It has two primary domains: RuvC-like and HNH, which cleave the two DNA strands, respectively. The double-stranded RNA complex binds to Cas9, forming a ribonucleoprotein (RNP) complex essential for precise gene editing. The RNP complex is stabilized through Cas9's specific domains binding to tracrRNA. The spacer sequence of crRNA is exposed and pairs complementarily with the target DNA sequence. The RNP complex recognizes and binds to the target DNA sequence through the spacer sequence of crRNA. The target DNA sequence must contain a Protospacer Adjacent Motif (PAM), essential for Cas9 recognition and binding. Once the crRNA pairs with the target DNA sequence, it forms an R-loop structure, a prerequisite for DNA cleavage by Cas9. Cas9 cleaves the double-stranded DNA at specific positions upstream of the PAM sequence, creating double-strand breaks (DSBs). The RuvC-like and HNH domains of Cas9 cleave the non-target and target strands, respectively. DSBs are repaired via non-homologous end joining (NHEJ) or homology-directed repair (HDR). NHEJ introduces random insertions or deletions (indels), leading to gene inactivation or loss of function, while HDR enables precise insertion or replacement of specific DNA fragments for accurate gene editing
Market Drivers for CRISPR Technology
In recent years, CRISPR technology has seen significant advancements in genome editing platforms, particularly in optimizing Cas9 proteins. For instance, optimized versions like eSpCas9 and SpCas9-HF have significantly reduced off-target effects and improved editing efficiency. These innovations have expanded CRISPR's applications across various fields. In basic research, high-precision CRISPR tools help scientists study gene functions, identify biomarkers, and discover drug targets. In clinical applications, reduced off-target effects enhance therapeutic safety, minimizing adverse reactions and providing more effective treatments. Additionally, optimized Cas9 proteins are useful for complex gene editing tasks such as multi-gene editing and large-scale genomic screening, driving CRISPR's broader adoption in research and medicine.
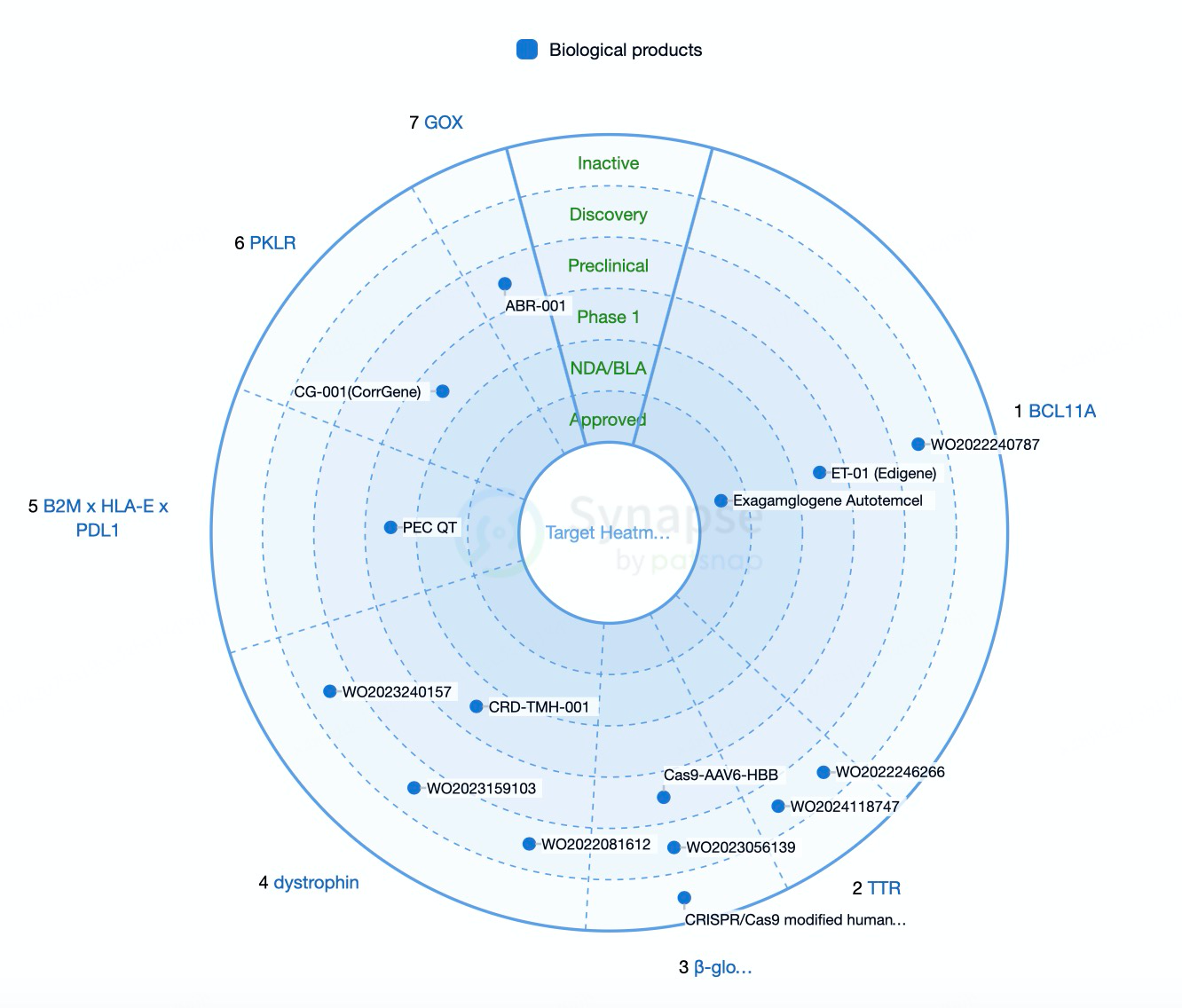
As the understanding of genetic diseases and cancer deepens, the demand for precision medicine and personalized therapies continues to grow. CRISPR technology, with its high efficiency and precision, has become a critical tool to meet these needs. Genetic diseases are often caused by specific gene mutations, and traditional therapies struggle to provide cures. In contrast, CRISPR technology enables precise gene editing to correct pathogenic mutations, offering curative treatment options. For instance, CRISPR has been successfully used in early-stage treatments for genetic disorders such as β-thalassemia and sickle cell disease.
In cancer therapy, CRISPR technology allows the editing of immune cells, such as CAR-T cells, enhancing their ability to recognize and eliminate cancer cells, thereby improving the efficacy of immunotherapy. Moreover, CRISPR is being used to develop novel gene therapies, including gene-editing vaccines and gene-drive systems, paving the way for new approaches in disease prevention and treatment. The increasing demand for CRISPR-based solutions not only drives innovation and technological advancement but also fosters the formation of related industrial chains, delivering significant economic and social benefits.
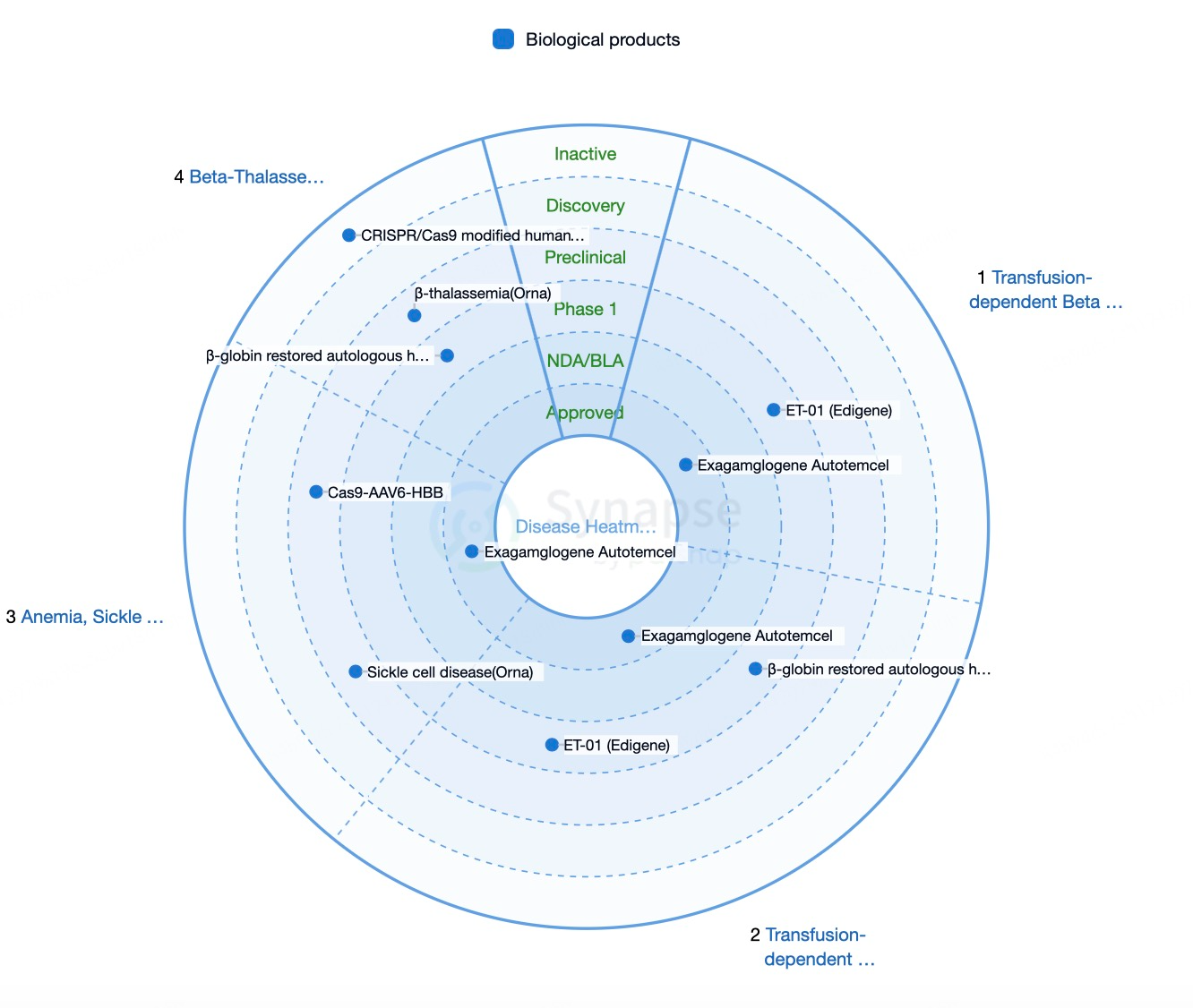
Regional Development of CRISPR Technology
The United States leads the research and development of CRISPR technology. Prominent institutions and biotech companies, such as Harvard University, MIT, the Broad Institute, CRISPR Therapeutics, and Intellia Therapeutics, are at the forefront of advancing this technology. The U.S. Food and Drug Administration (FDA) has approved several clinical trials for CRISPR-based therapies, targeting conditions such as sickle cell disease (SCD), transfusion-dependent β-thalassemia (TDT), and hereditary blindness. On December 8, 2023, the FDA approved Casgevy (exa-cel), a CRISPR-based gene-editing therapy developed by CRISPR Therapeutics and Vertex Pharmaceuticals, for treating SCD and TDT.
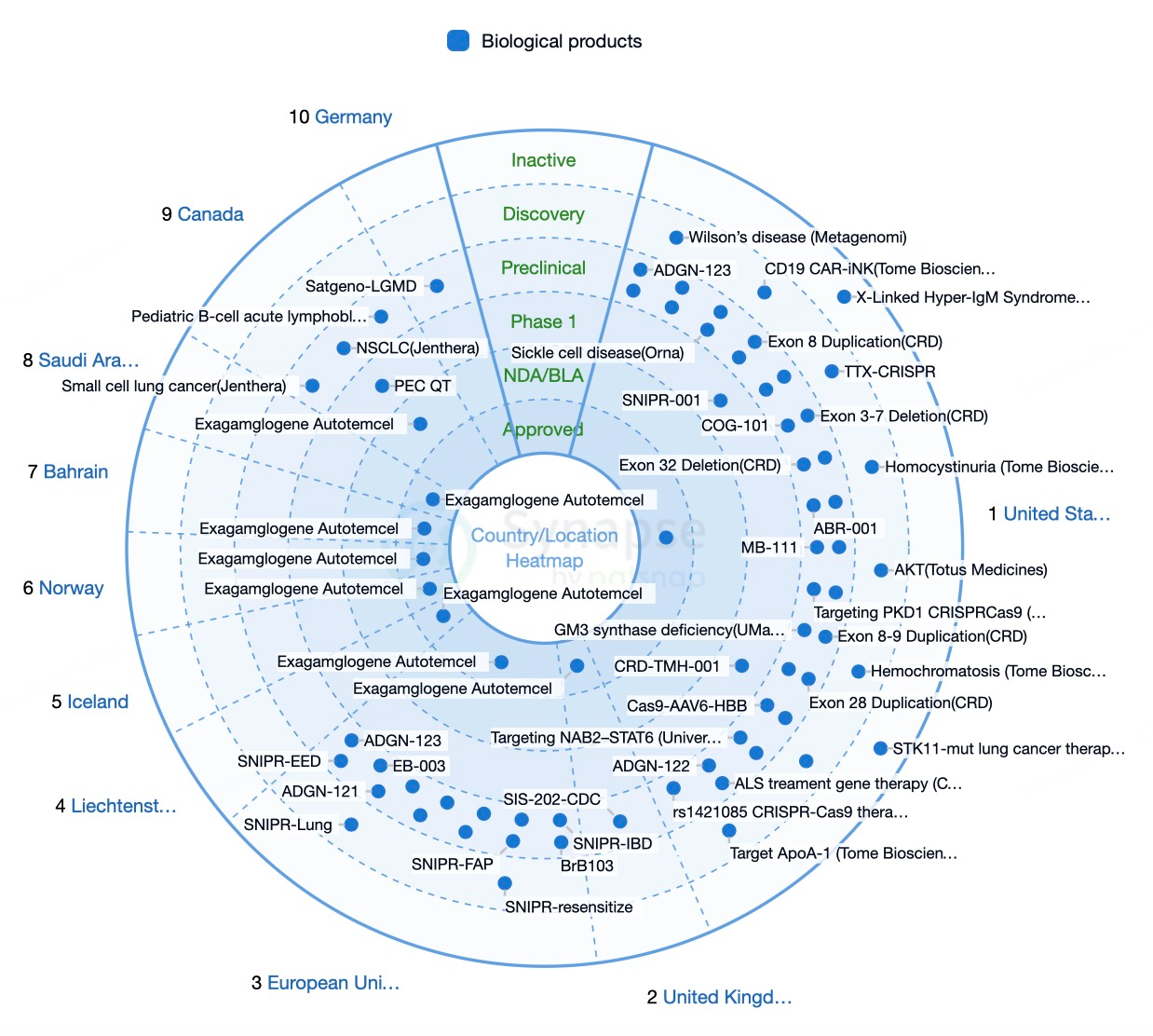
The United Kingdom is also at the forefront of CRISPR regulation and approvals. On November 16, 2023, the UK's Medicines and Healthcare products Regulatory Agency (MHRA) approved Casgevy (exa-cel) for treating SCD and TDT. Institutions like the University of Cambridge and Imperial College London have made significant contributions to the basic and applied research of CRISPR technology.
Several European Union countries are actively advancing CRISPR research and clinical applications. The European Medicines Agency (EMA) approved Casgevy (exa-cel) for treating SCD and TDT on December 15, 2023. Research institutions and biotech firms in Germany, France, and the Netherlands have achieved notable progress in clinical trials and applications of CRISPR technology.
Competitive Landscape of the CRISPR Gene Editing Therapy Market
CRISPR Therapeutics, based in Basel, Switzerland, is a leading gene editing company focused on utilizing CRISPR-Cas9 technology to treat various genetic disorders. Its flagship product, Casgevy (exa-cel), is a gene editing therapy for sickle cell disease (SCD) and transfusion-dependent β-thalassemia (TDT). This therapy received approval from both the FDA and EMA in 2023. The company is also advancing CTX001, an autologous cell therapy for β-thalassemia and SCD, which has demonstrated promising safety and efficacy in multiple clinical trials.
Intellia Therapeutics, headquartered in Cambridge, Massachusetts, specializes in the development and application of CRISPR-Cas9-based therapies. The company is advancing both in vivo and ex vivo gene editing treatments for various genetic diseases. Key products include NTLA-2001, an in vivo gene editing therapy for transthyretin amyloidosis (ATTR), which has shown significant efficacy and safety in clinical trials, and NTLA-2002, an in vivo therapy for hereditary angioedema (HAE), which has received FDA approval for clinical trials and demonstrated positive results in early-stage studies.
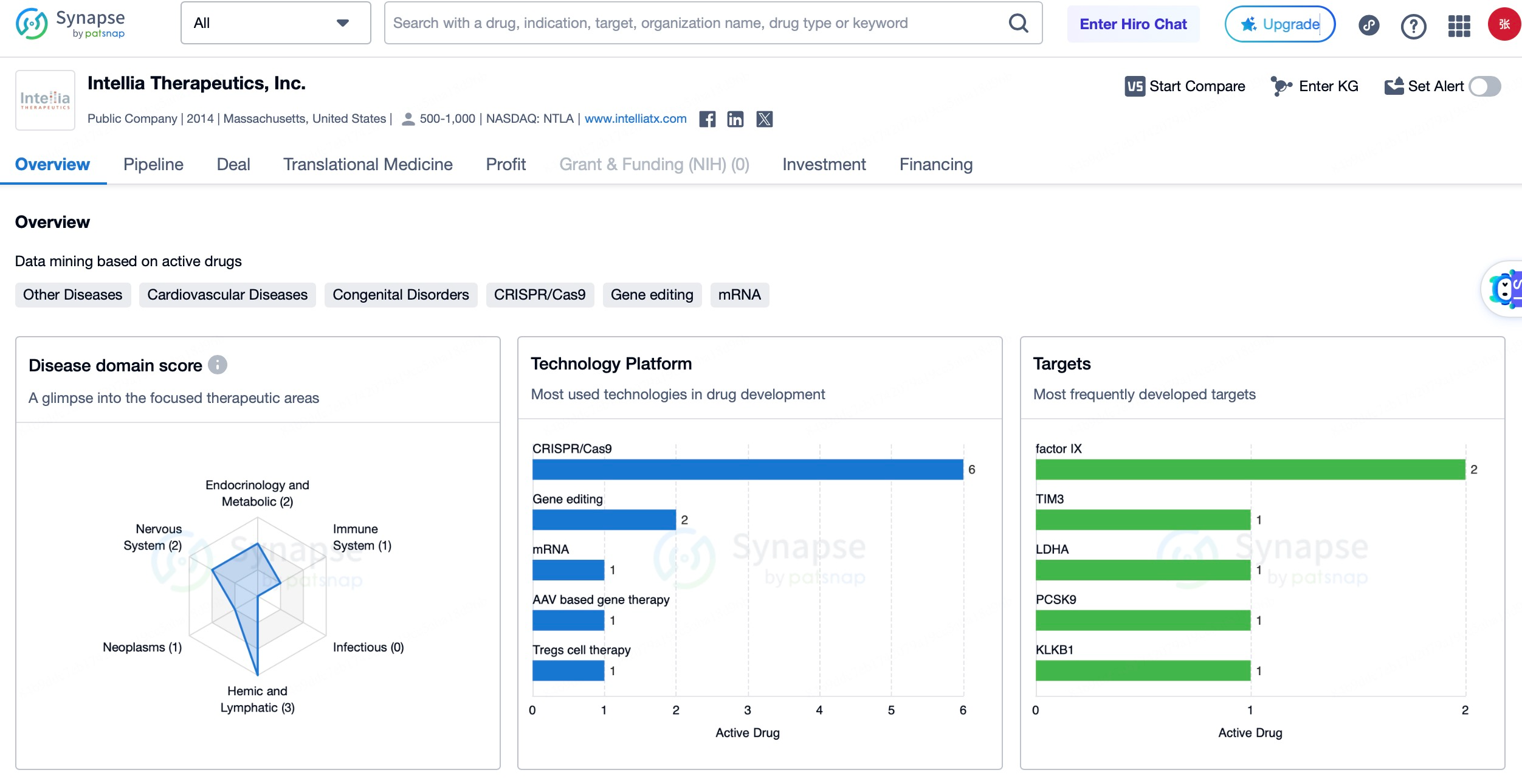
Editas Medicine, also based in Cambridge, Massachusetts, is a prominent player in the gene editing field, leveraging CRISPR-Cas9 technology to address genetic diseases. Its lead product, EDIT-101, is an in vivo gene editing therapy for Leber congenital amaurosis type 10 (LCA10). Using an AAV5 vector to deliver the CRISPR-Cas9 system, this therapy targets pathogenic mutations in the CEP290 gene to restore photoreceptor function. It has shown good safety and preliminary efficacy in Phase 1/2 clinical trials. Another key product, EDIT-301, treats SCD and TDT by editing hematopoietic stem cells to produce high levels of fetal hemoglobin, reducing or eliminating disease symptoms. EDIT-301 is currently under evaluation in several clinical trials, showing favorable safety and efficacy.
Mammoth Biosciences, located in San Francisco, California, was co-founded by CRISPR pioneer Dr. Jennifer Doudna. The company focuses on applying CRISPR technology for disease diagnosis and gene editing. Notable offerings include the DETECTR platform, a CRISPR-based diagnostic tool that leverages Cas enzymes and guide RNA to deliver genetic diagnostic results within 20 minutes. This platform gained prominence during the COVID-19 pandemic through the RADx initiative and collaborations with the U.S. Department of Defense, showcasing its potential in public health emergencies. Mammoth is also developing gene editing therapies for genetic disorders using its proprietary ultra-small Cas enzymes (e.g., Cas14 and Cas12), which are smaller, more thermally stable, and exhibit faster reaction kinetics, enabling efficient delivery and gene editing. Collaborations with companies like Bayer and Regeneron are accelerating Mammoth’s therapeutic development efforts.
Beam Therapeutics, headquartered in Cambridge, Massachusetts, is a biotechnology company specializing in base editing technology. This approach enables precise genetic modifications, providing tailored treatments for genetic disorders. Its key products include BEAM-101 and BEAM-102. BEAM-101 is an ex vivo gene-editing therapy designed for the treatment of sickle cell disease (SCD). It works by editing hematopoietic stem cells to increase fetal hemoglobin (HbF) levels, thereby mitigating or eliminating SCD symptoms. BEAM-101 has received FDA IND approval and is currently undergoing the Phase 1/2 BEACON-101 clinical trial. Early data indicate its ability to significantly elevate HbF levels while reducing HbS levels, demonstrating both safety and efficacy. BEAM-102 targets transfusion-dependent β-thalassemia (TDT), similarly using edited hematopoietic stem cells to increase HbF levels and alleviate or eliminate disease symptoms. BEAM-102 is currently in the IND feasibility study phase, laying the groundwork for future clinical trials.
Summary
The market outlook for CRISPR technology is promising, with expanding applications in gene editing, gene therapy, and immunotherapy. Advances in technical optimization, safety improvements, growing market demand, and supportive policies are driving the rapid development of CRISPR technologies. As more clinical trials succeed and regulatory approvals increase, CRISPR holds the potential to offer precise and effective treatments for genetic disorders and cancers. This progress is also expected to stimulate the growth of related industries, delivering positive economic and social impacts.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference:
1.Wang, S.W., et al., Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol Cancer, 2022. 21(1): p. 57.
2.Liu, Z., et al., Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy. Mol Cancer, 2023. 22(1): p. 35.