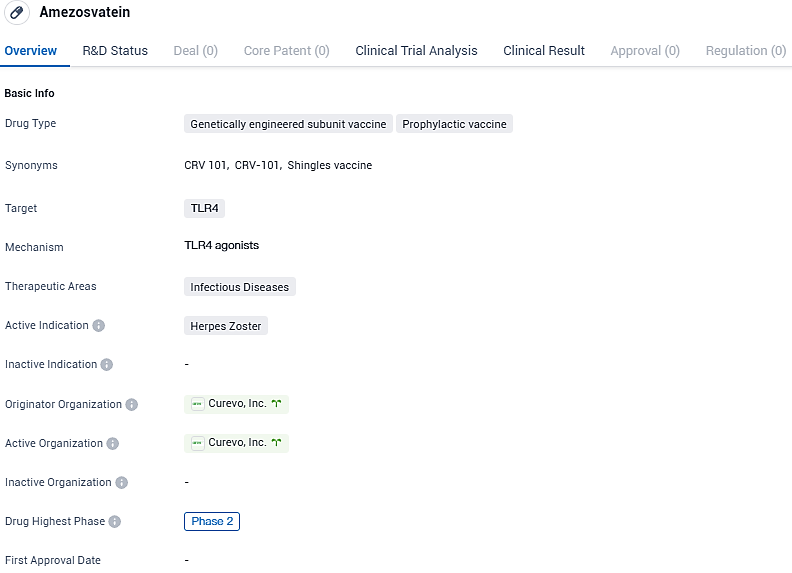
Curevo Vaccine's Phase 2 study shows Amezosvatein, a new shingles vaccine, is effective compared to Shingrix
Curevo Vaccine, an independent biotech firm specializing in the advancement of vaccines targeting the varicella zoster virus, aiming for enhanced safety and wider distribution, has released encouraging results from a Phase 2 study involving 876 subjects. This trial evaluated the effectiveness of amezosvatein (also identified as CRV-101, a non-mRNA based, adjuvanted subunit vaccine) in a direct comparison with Shingrix among individuals aged 50 and above.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the double-blind, placebo-controlled second-stage clinical study, Amezosvatein achieved all its main goals, displaying parity with Shingrix by inducing comparable antibody-mediated immune reactions. Additionally, the trial noted a reduced incidence of reported adverse reactions, both locally and systemically for Amezosvatein. Curevo is poised to propel Amezosvatein into the international Phase 3 trials come 2024, targeting the substantial shingles immunization sector now surpassing $4 billion.
Regarding the study's chief immune-response goal assessed one month post-second inoculation, Amezosvatein evoked immune reactions comparable to those triggered by Shingrix. Furthermore, the Vaccine Response Rate for Amezosvatein reached 100.0%, while Shingrix recorded a slightly lower 97.9%.
Amezosvatein surpassed another major goal in safety, revealing fewer documented local and systemic adverse reactions compared to prior studies. This contrast was particularly noticeable in the reduced occurrence of more severe reactions within a week following the vaccinations. Should these findings be reproduced in the subsequent Phase 3 trial, Amezosvatein may offer a substantial benefit by lessening the impact of severe adverse events that could disrupt routine life.
Curevo's Chief Medical Officer, Dr. Guy De La Rosa, expressed concerns about the low uptake of Shingrix despite years on the market, attributing this to tolerability issues that fuel vaccine reluctance and the avoidance of a second shot. “Amezosvatein has been crafted not only for high efficacy but also for market-leading tolerability. The data from the Phase 2 study is highly promising," he highlighted.
Curevo's CEO, George Simeon, pointed out that less than 5% of qualified adults across major European nations and China have completed their Shingrix regimen, while a significant majority of American adults have yet to receive a shingles shot. He outlined the substantial and predominantly unmet market potential in shingles, with only a small portion of the larger $350 billion addressable global market successfully targeted. Simeon emphasized Curevo's commitment to speedily deliver Amezosvatein across international markets.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
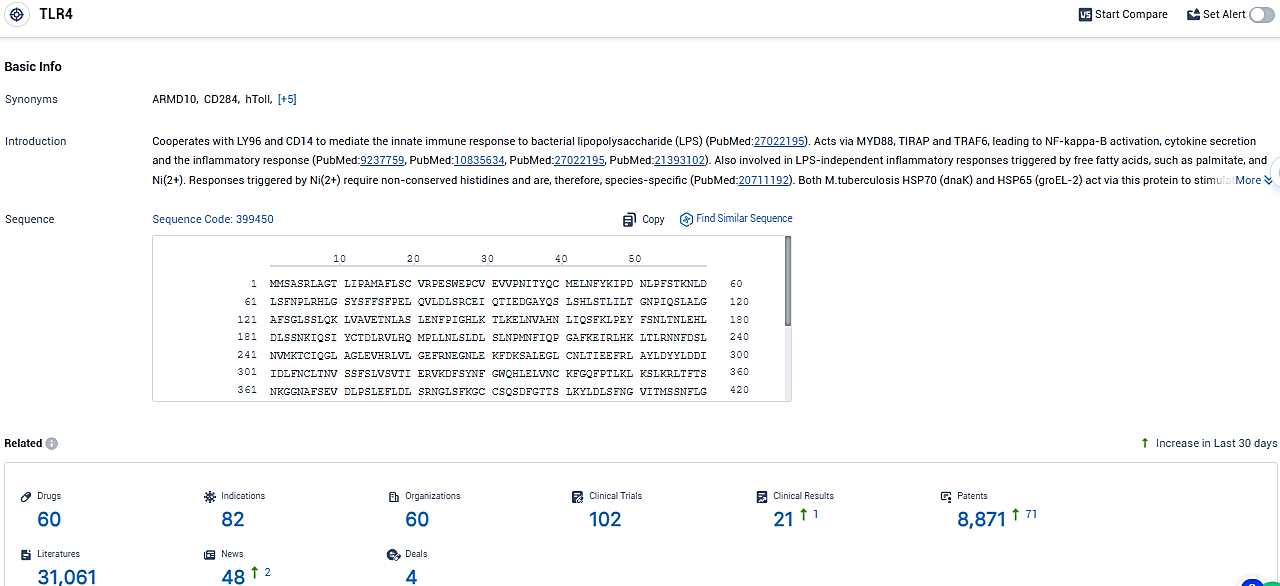
According to the data provided by the Synapse Database, As of January 12, 2024, there are 60 investigational drugs for the TLR4 tagets, including 82 indications, 60 R&D institutions involved, with related clinical trials reaching 102, and as many as 8871 patents.
Amezosvatein is the assigned non-proprietary name for CRV-101, a non-mRNA adjuvanted subunit vaccine under investigation by Curevo. Like Shingrix, amezosvatein uses an adjuvant targeting the TLR4 pathway to boost the immune response to the gE antigen. Amezosvatein was engineered to have a best-in-class safety profile in addition to manufacturing advantages to improve vaccine accessibility.