Decoding Carboprost Tromethamine: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Carboprost Tromethamine's R&D Progress
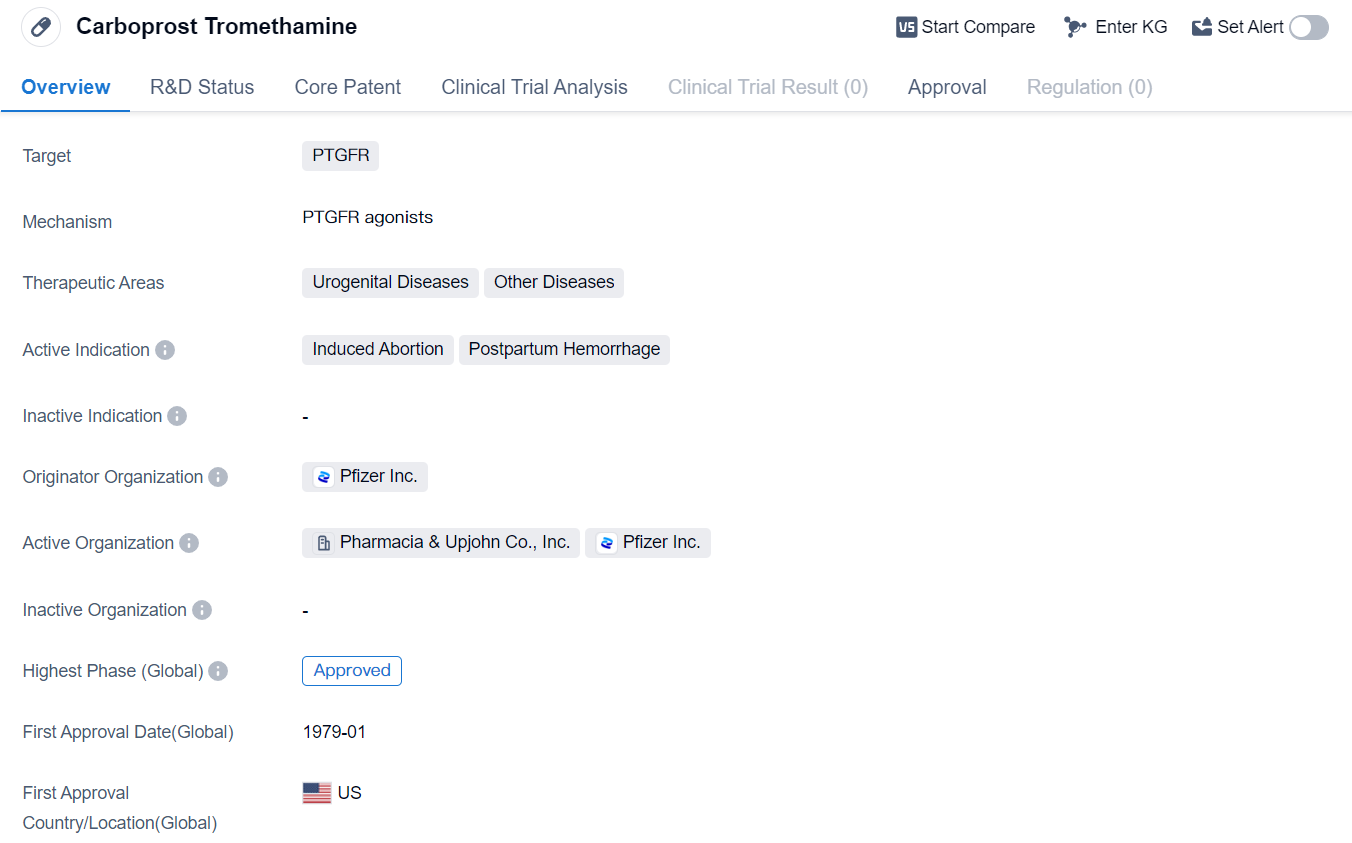
Carboprost Tromethamine is a small molecule drug that targets the PTGFR (Prostaglandin F Receptor) and is used in the treatment of urogenital diseases and other diseases. Its active indications include induced abortion and postpartum hemorrhage. The drug was developed by Pfizer Inc., a renowned pharmaceutical company.
Carboprost Tromethamine has been approved for use in the global market . Its first approval date in the global market was in January 1979, with the United States being the first country/location to approve its use.
As a small molecule drug, Carboprost Tromethamine is designed to interact with the PTGFR, a receptor involved in various physiological processes. By targeting this receptor, the drug aims to provide therapeutic benefits in the treatment of urogenital diseases and other related conditions.
One of the active indications for Carboprost Tromethamine is induced abortion. This suggests that the drug may be used to terminate pregnancies in a safe and controlled manner. Additionally, the drug is also indicated for postpartum hemorrhage, a condition characterized by excessive bleeding after childbirth. By effectively managing postpartum hemorrhage, Carboprost Tromethamine can potentially reduce complications and improve patient outcomes.
Pfizer Inc., the originator organization of Carboprost Tromethamine, is a well-established pharmaceutical company known for its contributions to the healthcare industry. The drug has reached the highest phase of development which is approved globally.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Carboprost Tromethamine: PTGFR agonist
PTGFR agonists are a type of drug that activate the prostaglandin F receptor (PTGFR) in the body. Prostaglandin F is a type of hormone-like substance that plays a role in various physiological processes, including inflammation, smooth muscle contraction, and reproductive functions.
From a biomedical perspective, PTGFR agonists can be used to modulate the activity of the PTGFR and influence its downstream signaling pathways. By binding to and activating the PTGFR, these agonists can elicit specific biological responses, such as reducing inflammation or promoting smooth muscle relaxation.
PTGFR agonists have potential therapeutic applications in various conditions. For example, in the field of reproductive medicine, they may be used to induce labor or control postpartum hemorrhage by stimulating uterine contractions. In the context of inflammation, PTGFR agonists can help regulate the immune response and alleviate symptoms associated with inflammatory diseases.
It's important to note that the specific effects and applications of PTGFR agonists may vary depending on the particular drug and the target tissue or organ. Therefore, the use of these agonists should be carefully evaluated and monitored by healthcare professionals to ensure their safety and efficacy.
Drug Target R&D Trends for Carboprost Tromethamine
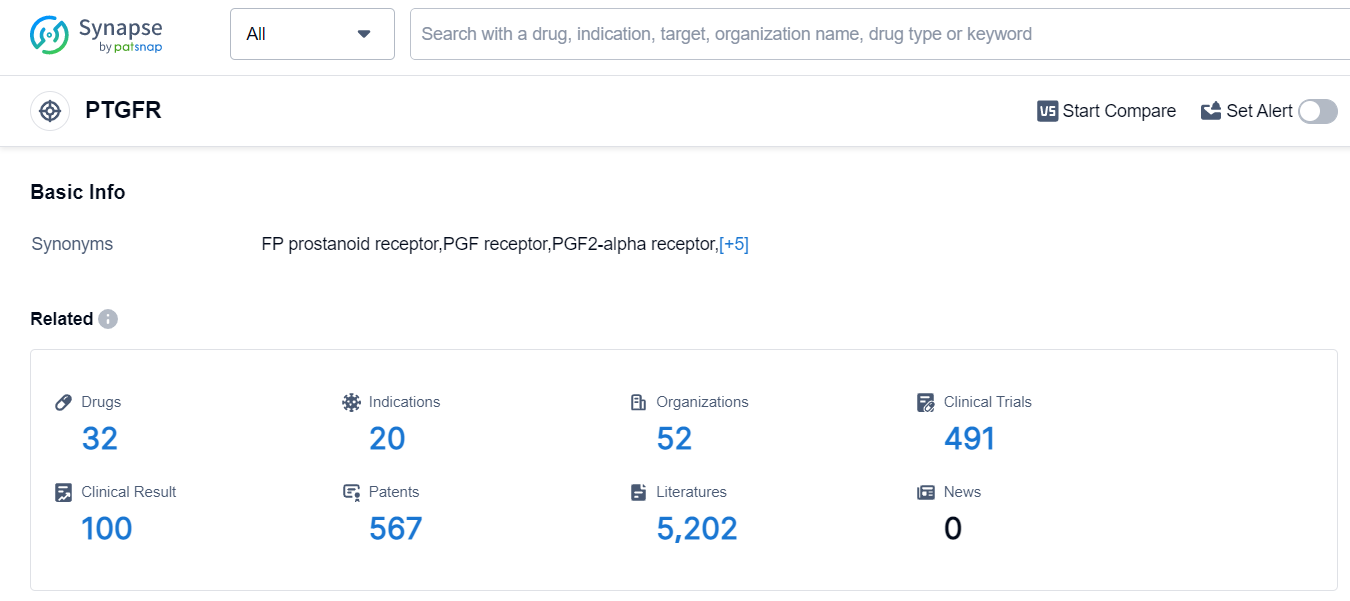
The analysis of the target PTGFR reveals a competitive landscape with multiple companies actively involved in research and development. Santen Pharmaceutical Co., Ltd. stands out with the highest number of approved drugs and drugs in advanced phases of development. Ocular Hypertension, Glaucoma, and Glaucoma, Open-Angle are the primary indications for which drugs have been approved. Small molecule drugs dominate the development landscape, indicating intense competition and a focus on innovation. Japan, the United States, and the European Union are leading in terms of approved drugs, with China also making progress. The future development of the target PTGFR holds promise for advancements in the treatment of eye-related conditions.
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 32 PTGFR drugs worldwide, from 52 organizations, covering 20 indications, and conducting 491 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Carboprost Tromethamine is a small molecule drug developed by Pfizer Inc. It targets the PTGFR and is used in the treatment of urogenital diseases and other diseases. Its active indications include induced abortion and postpartum hemorrhage. The drug has been approved for use in the global markets, with its first approval dating back to January 1979 in the United States. Carboprost Tromethamine offers potential therapeutic benefits in managing urogenital diseases and postpartum hemorrhage, providing a valuable treatment option for patients in need.