Decoding Diacerein: A Comprehensive Study of its R&D Trends
Diacerein's R&D Progress
Diacerein is a small molecule drug that targets IL-1β, a protein involved in the immune response. It has been approved for use in various therapeutic areas, including immune system diseases, congenital disorders, endocrinology and metabolic disease, as well as skin and musculoskeletal diseases. The drug has shown efficacy in treating rheumatoid arthritis, osteoarthritis, epidermolysis bullosa simplex, pemphigoid, bullous, gout, and diabetes mellitus type 2.
The originator organization of Diacerein is Proter Srl, an Italian company. The drug received its first approval in Italy in December 1986, making it one of the early drugs in the market. Diacerein has also obtained approval in other countries globally, indicating its widespread acceptance and recognition.
One notable aspect of Diacerein is its regulatory status as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives and benefits to the manufacturer, such as market exclusivity and financial support, to encourage the development of drugs for rare diseases.
Overall, Diacerein has established itself as a valuable therapeutic option for various medical conditions. Its approval in multiple countries and its orphan drug status highlight its effectiveness and potential in addressing unmet medical needs. As a small molecule drug targeting IL-1β, Diacerein offers a targeted approach to modulating the immune response, which can be beneficial in managing immune-related diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Diacerein: IL-1β inhibitors
IL-1β inhibitors are a type of medication that target and inhibit the activity of interleukin-1 beta (IL-1β), which is a pro-inflammatory cytokine. Interleukin-1 beta plays a crucial role in the inflammatory response and is involved in various diseases, including autoimmune disorders, inflammatory conditions, and certain types of cancer.
From a biomedical perspective, IL-1β inhibitors are designed to specifically block the action of IL-1β by binding to its receptors or inhibiting its production. By doing so, these inhibitors can help reduce inflammation and alleviate symptoms associated with IL-1β-mediated diseases.
IL-1β inhibitors are used in the treatment of conditions such as rheumatoid arthritis, systemic juvenile idiopathic arthritis, and certain autoinflammatory diseases. They can help control inflammation, relieve pain, and improve overall quality of life for patients.
It is important to note that IL-1β inhibitors are prescription medications and should only be used under the guidance and supervision of a healthcare professional. The specific dosage and duration of treatment may vary depending on the individual and the condition being treated. Regular monitoring and follow-up with a healthcare provider are essential to ensure the safe and effective use of IL-1β inhibitors.
Drug Target R&D Trends for Diacerein
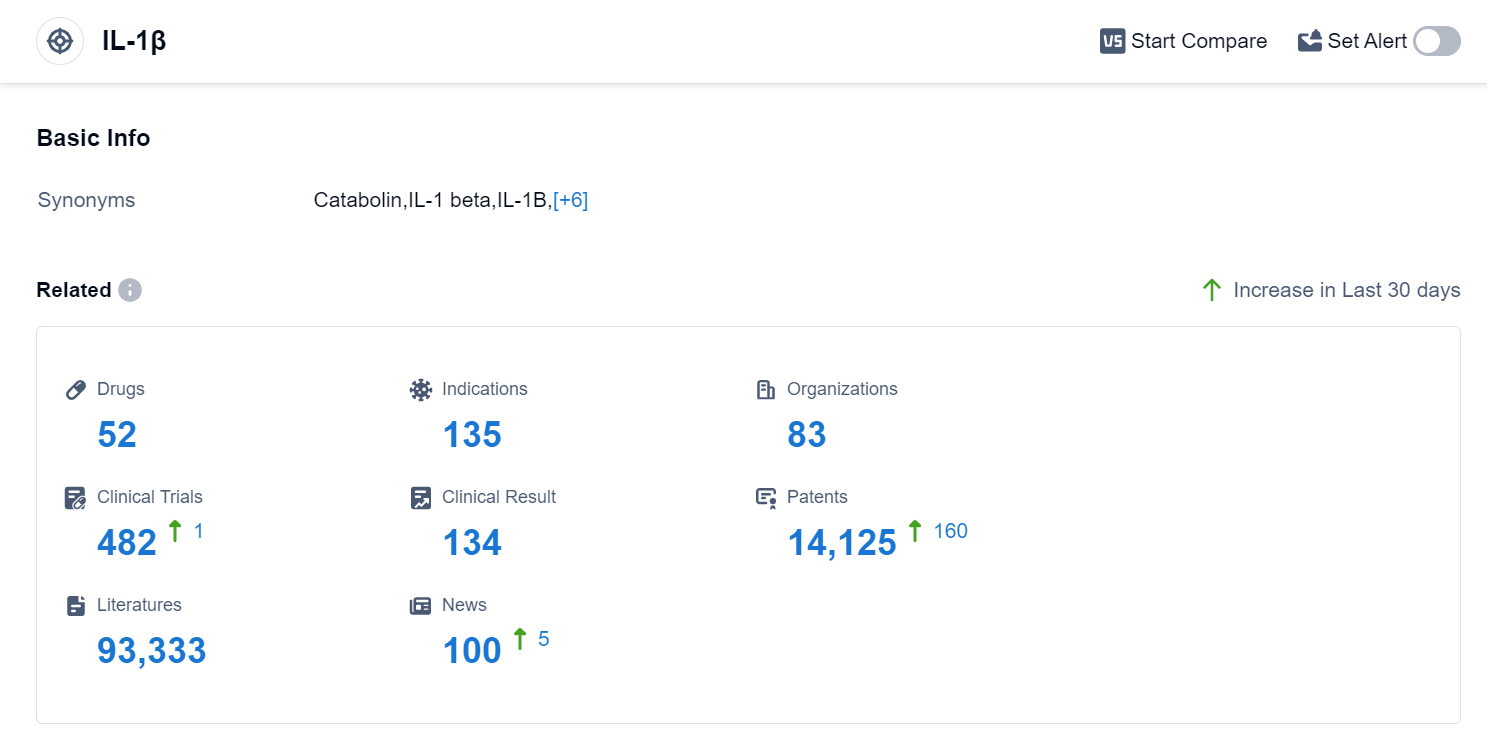
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 52 IL-1β drugs worldwide, from 83 organizations, covering 135 indications, and conducting 482 clinical trials.
The analysis of the target IL-1β in the pharmaceutical industry reveals that Novartis AG is the company with the highest stage of development, with drugs in various phases. Drugs under this target have been approved for indications such as osteoarthritis, rheumatoid arthritis, gout, and cardiovascular diseases. Small molecule drugs, monoclonal antibodies, and fusion proteins are progressing rapidly, with biosimilars indicating intense competition. The development of drugs under this target is taking place in various countries, including China. Overall, the competitive landscape for target IL-1β is dynamic, with potential for future growth and advancements in the field.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Diacerein is a small molecule drug developed by Proter Srl that targets IL-1β. It has been approved for use in various therapeutic areas and has shown efficacy in treating conditions such as rheumatoid arthritis, osteoarthritis, and diabetes mellitus type 2. With its first approval in Italy in 1986, Diacerein has a long-standing presence in the market and has obtained approvals in multiple countries globally. Its orphan drug status further emphasizes its importance in addressing rare diseases. Overall, Diacerein is a valuable drug in the field of biomedicine, offering targeted treatment options for a range of medical conditions.