Decoding Floxuridine: a comprehensive study of its R&D trends and its clinical results in 2024 ASCO_GI
On 18 Jan 2024, the phase II trial of induction systemic mFOLFIRINOX followed by hepatic arterial infusion of floxuridine and dexamethasone given concurrently with systemic mFOLFIRI as a first-line therapy in patients with unresectable liver-dominant intrahepatic cholangiocarcinoma (HELIX-1) was presented in 2024 ASCO_GI.
Floxuridine's R&D Progress
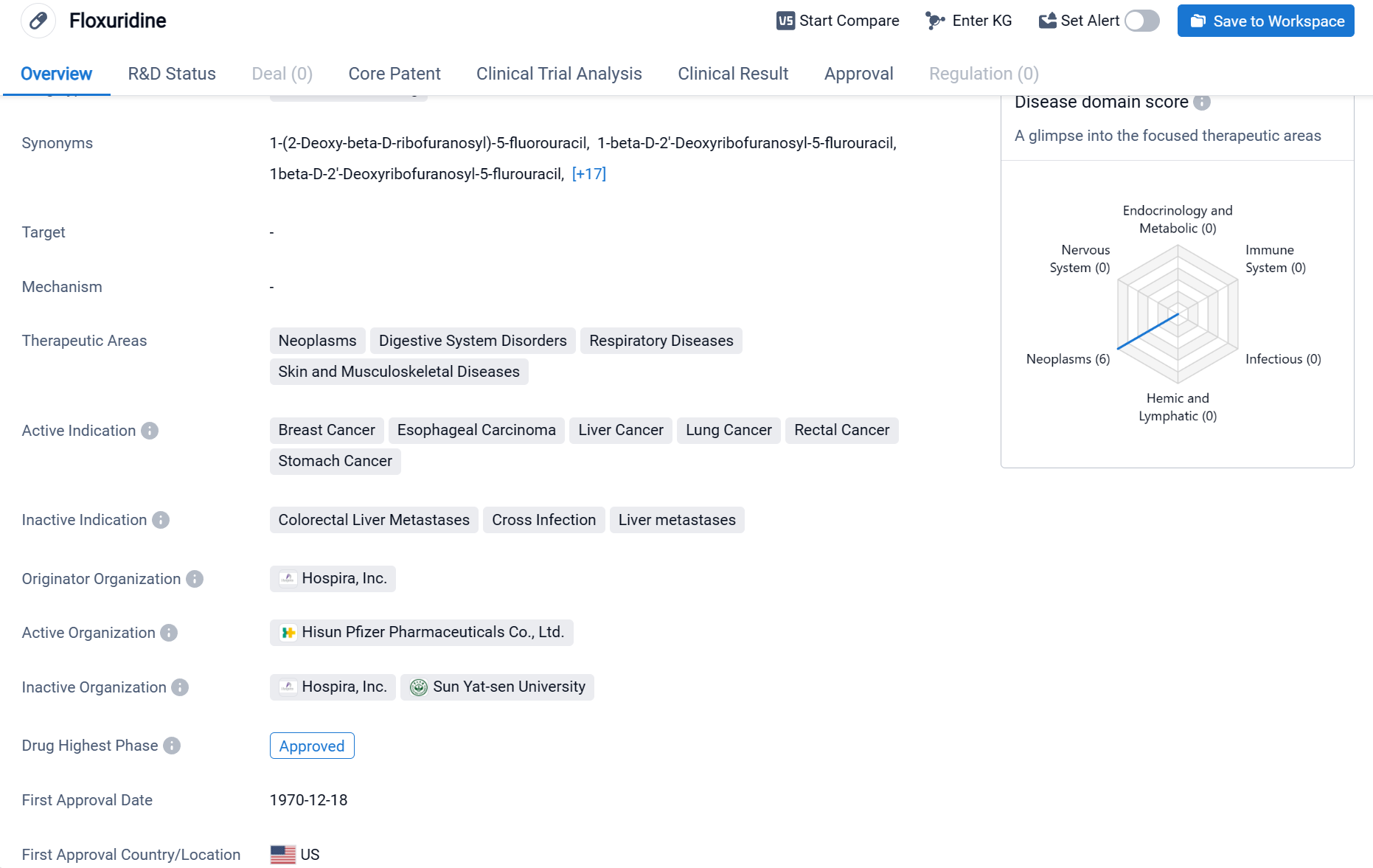
Floxuridine is a small molecule drug that falls under the therapeutic areas of neoplasms, digestive system disorders, respiratory diseases, and skin and musculoskeletal diseases. It is primarily indicated for the treatment of breast cancer, esophageal carcinoma, liver cancer, lung cancer, rectal cancer, and stomach cancer.
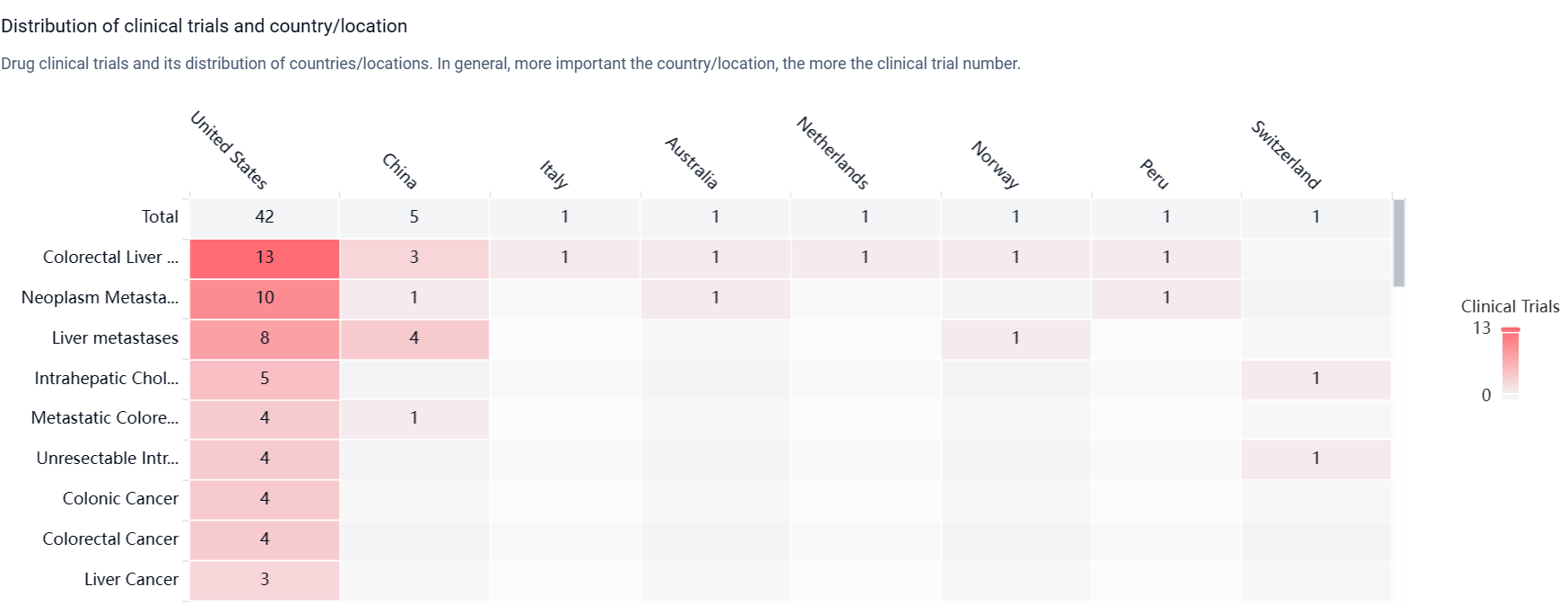
According to the Patsnap Synapse, Floxuridine has received approval for use in various countries. And the clinical trial distributions for Floxuridine are primarily in the United States, China and Italy. The key indication is Colorectal Liver Metastases.
Detailed Clinical Result of Floxuridine
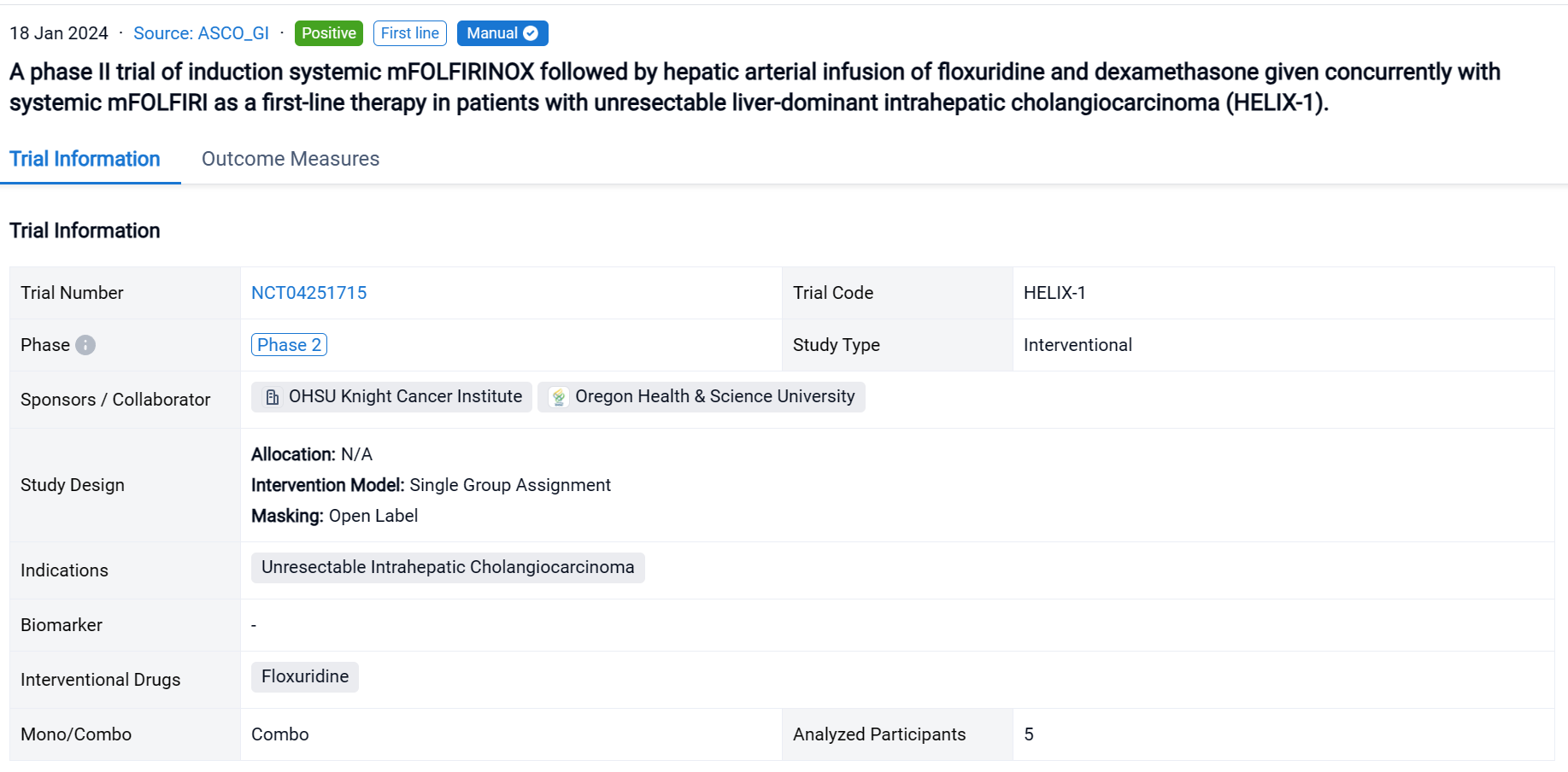
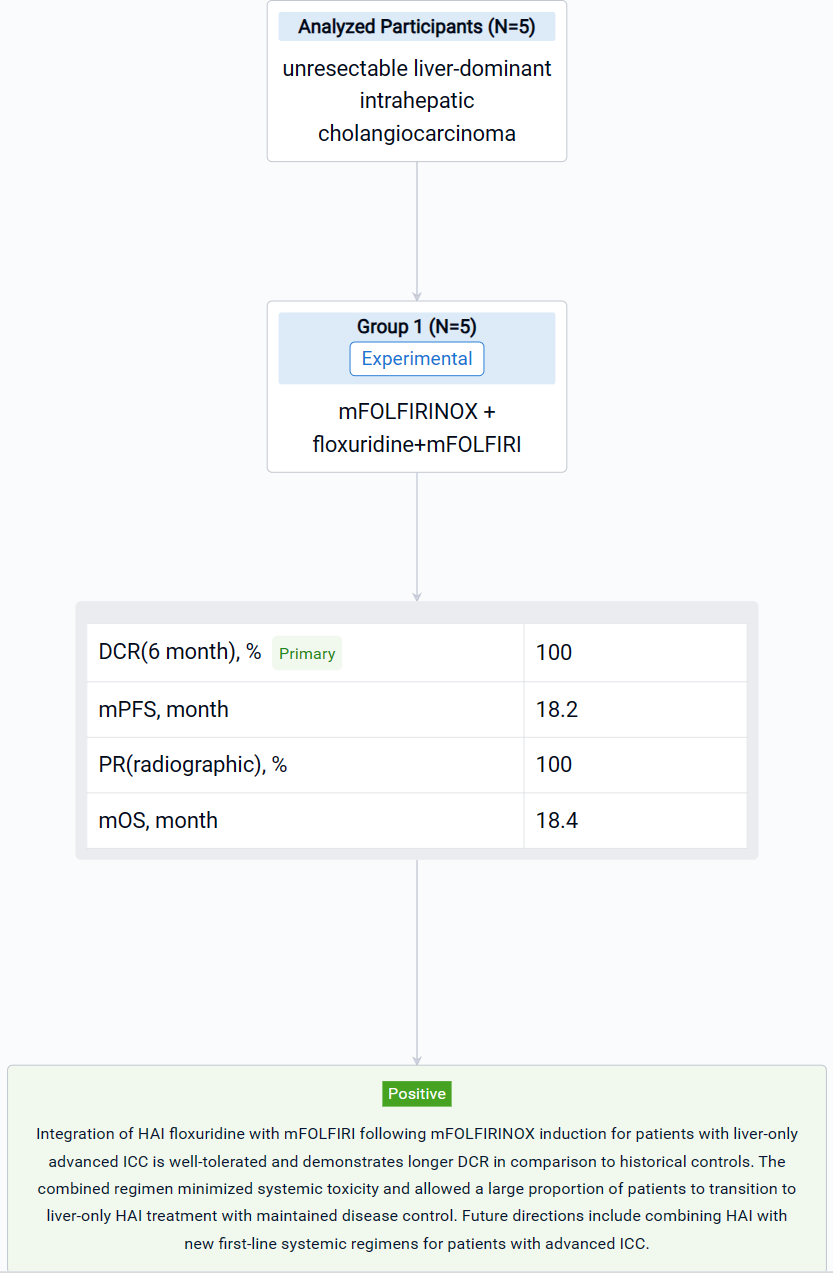
This investigator-initiated, first-line, single-center, single-arm phase II clinical trial (NCT04251715) was designed with a patient safety run-in evaluating toxicity from the combined therapy.
In this study, eligible patients were treated with systemic mFOLFIRINOX for 4 cycles in order to select those likely to benefit from HAI. Patients with disease control on restaging proceeded to HAI pump placement and treatment with HAI floxuridine for 14 days followed by systemic mFOLFIRI on a 28-day cycle. The co-primary objectives were to assess safety of the combined therapeutic strategy and the disease control rate (DCR) at 6 months (RECIST v1.1) at end of trial (EOT).
The result showed that a total of n=5 patients with liver-only ICC enrolled in the trial and completed the entire study protocol. The median age was 60 years (range 42-69) with a dominant lesion size of 9.8cm (range 8.4-14.5). All patients had both right and left hemiliver involvement with a median of 9 intrahepatic tumors (range 1-15). No patients experienced grade 3 or 4 adverse events, or hepatic dysfunction leading to cessation of HAI therapy. The DCR at 6 months was 100%, with a mPFS of 18.2 months. All five patients achieved partial radiographic response (PR) while receiving HAI therapy, and remain alive with liver-only disease at a median of 18.4 months (range 12.7-20) after study enrollment. After continuing treatment with HAI and systemic mFOLFIRI beyond the EOT, two patients transitioned to HAI treatment only and then to biochemical and radiographic surveillance without therapy after demonstrating PR and CA19-9 normalization.
It can be concluded that Integration of HAI floxuridine with mFOLFIRI following mFOLFIRINOX induction for patients with liver-only advanced ICC is well-tolerated and demonstrates longer DCR in comparison to historical controls. The combined regimen minimized systemic toxicity and allowed a large proportion of patients to transition to liver-only HAI treatment with maintained disease control. Future directions include combining HAI with new first-line systemic regimens for patients with advanced ICC.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
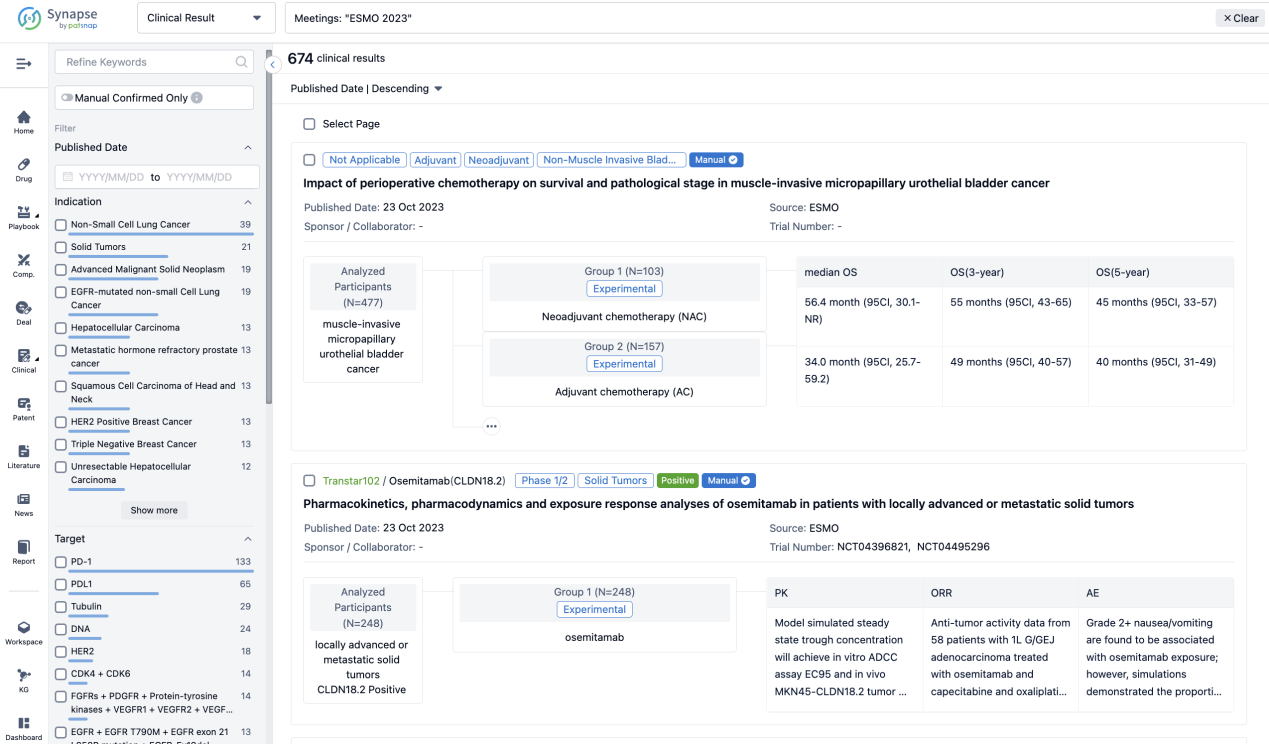
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

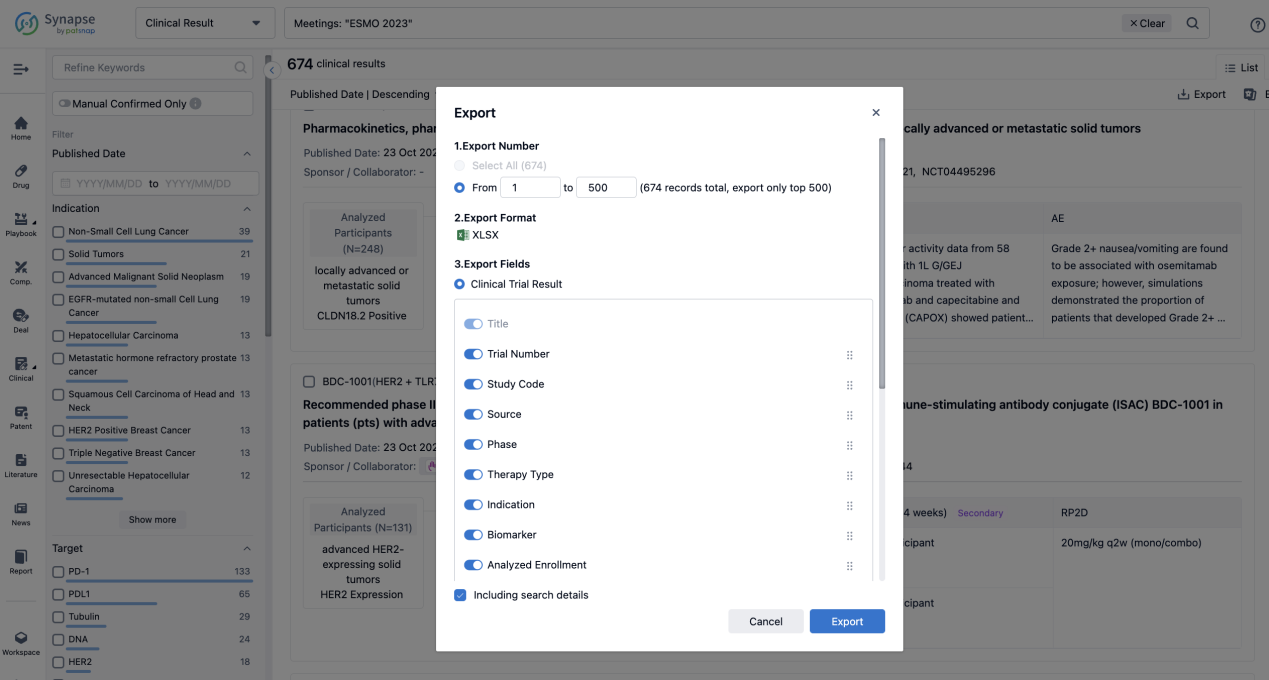
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!