Decoding Orca-T: a comprehensive study of its R&D trends and its clinical results in 2023 ASH
The interim results of the single center phase I trial evaluating the T cell reduced Orca-T cell therapy product and single agent tacrolimus in the RIC setting will be reported in the 2023 ASH Congress,demonstrating its potential benefits.
Orca-T's R&D Progress
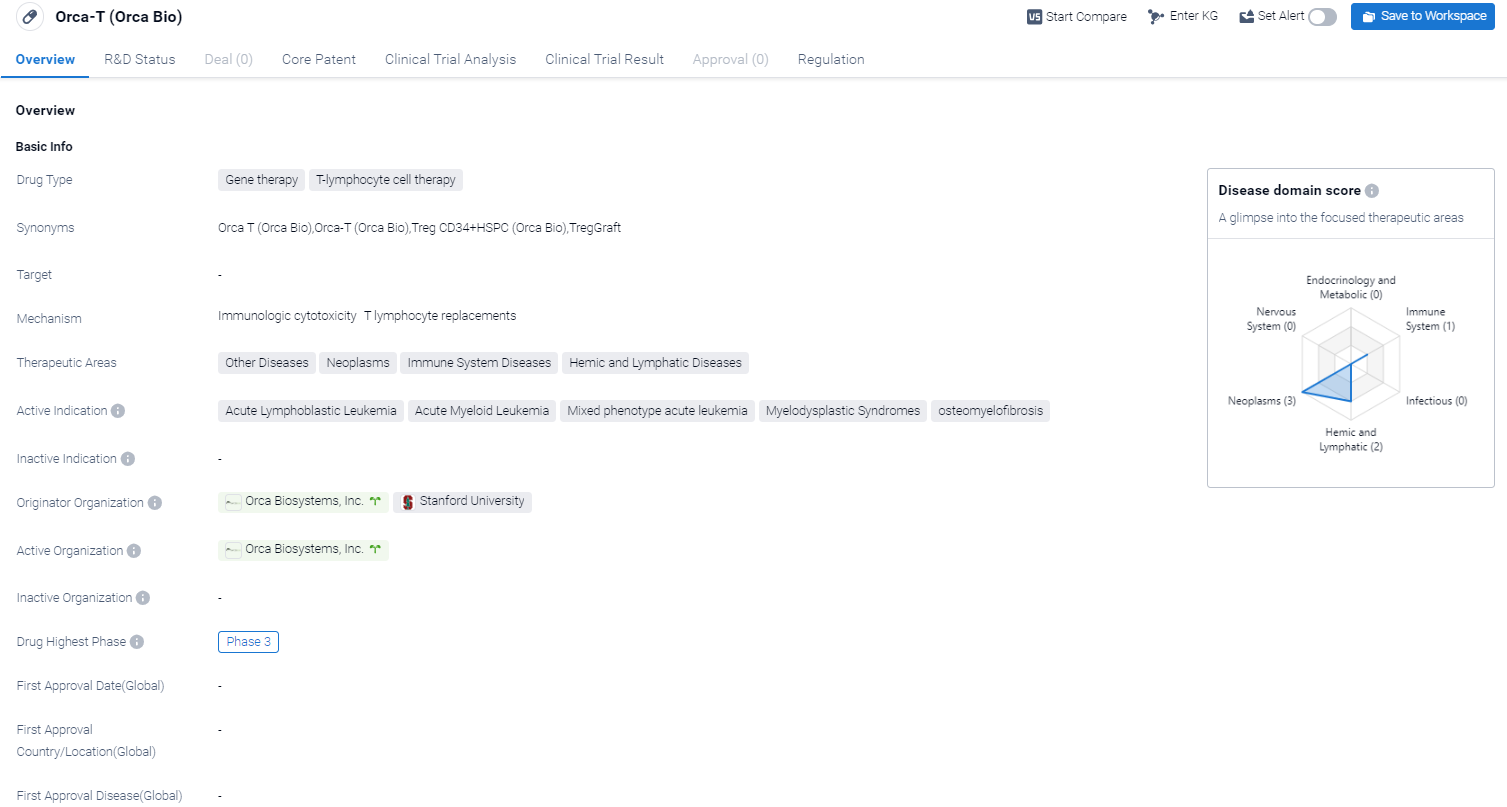
Orca-T is a gene therapy and T-lymphocyte cell therapy drug that falls under the category of regenerative medicine advanced therapy. It is being developed by Orca Biosystems, Inc. in collaboration with Stanford University. Orca-T is primarily targeted towards the treatment of various diseases within the therapeutic areas of other diseases, neoplasms, immune system diseases, and hemic and lymphatic diseases.
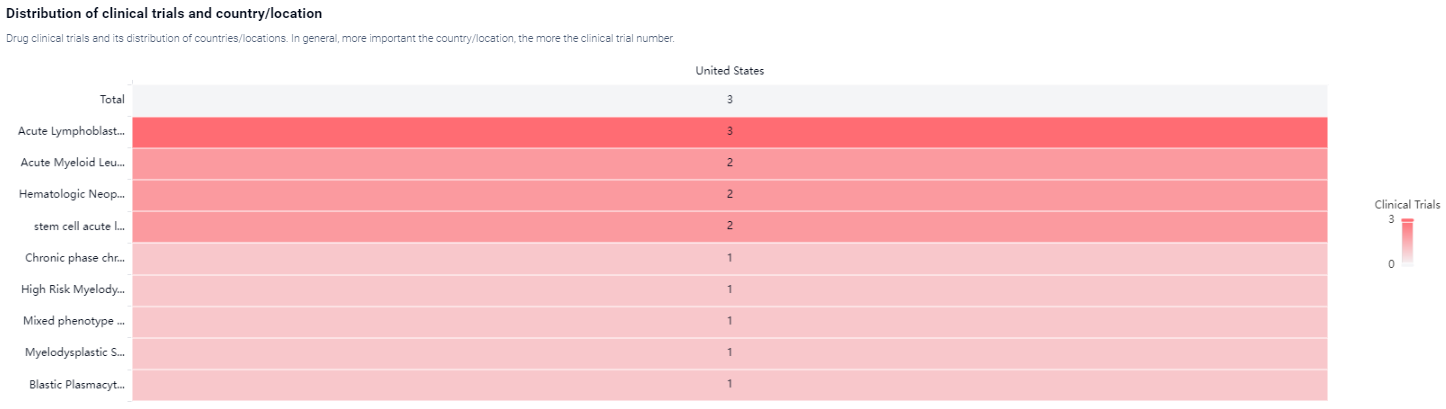
According to the Patsnap Synapse, Orca-T is currently in Phase 3, which is the highest phase of clinical development. And the clinical trial area for Orca-T is primarily in the United States. The key indication is Acute Lymphoblastic Leukemia.
Detailed Clinical Result of Orca-T
This trial is a single-center open-label phase 1 feasibility and safety study for dose escalation of conventional T cell (Tcon) infusion.
In this study, primary objectives included measuring the incidence of grade III-IV acute GVHD, time of engraftment and donor T cell chimerism at day +30. Secondary objectives are measuring relapse free survival, severity of GVHD and incidence of serious infections. While the study has three independent trial arms depending on donor type (HLA matched related and unrelated, 9/10 HLA matched or haploidentical matched), accrual has focused on the HLA-matched cohort which is reported here. The first 11 patients on this arm were conditioned with fludarabine 40mg/m2, melphalan 50 mg/m2 and 4 Gy of total body irradiation (TBI), an additional 4 patients have been treated with thiotepa 10 mg/kg replacing the melphalan. All patients received single agent tacrolimus with a target goal of 6-8 ng/ml.
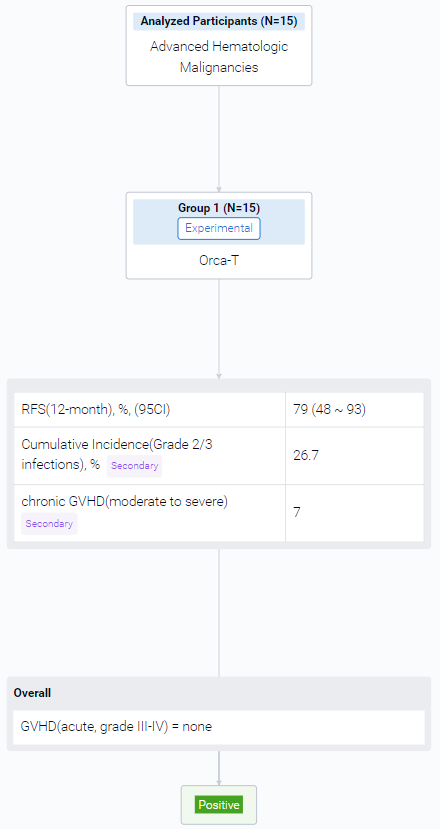
The result showed that a total of 15 HLA-matched patients have been recruited thus far in this interim dose escalation analysis. The median patient age is 68.0 years (range 62 to 72), with 73% male (11/15) patient distribution. Pre transplant, 53% had acute myeloid leukemia (8/15), 20% had myelodysplastic syndrome (3/15), 13% had acute lymphoblastic leukemia (2/15) and 13% had a myeloproliferative neoplasm (2/15). Prior to transplant, all acute leukemia patients were in complete remission (CR) or CR with incomplete count recovery. Median follow up was 10.4 months for the entire cohort (range 2 to 20 months). 14/15 patients had engraftment by day 20 and 1/15 at day 39 post-transplant. Based on these findings, no dose escalation of Tcon infusion was needed. None of the patients had infusion reactions on day of transplant nor engraftment syndrome. Median percent donor chimerism for CD15 was high, with 100% at day 30 (15/15, range 94% to 100%) and 99% (13/15, patients with available data, range 98% to 100%) at day 90. Median CD3 chimerism at days 30 and 90 of transplant were also high at 99% (15/15, range 68% to 100%) and 99% (for 13/15 patients with available data, range 78% to 100%) respectively. No patients developed grade III-IV acute GVHD (Fig.), defined <100 days post-transplant. One patient developed grade II aGVHD. In 12 months, the incidence for moderate to severe chronic GVHD was 7%. The 12-month relapse free survival (RFS) is thus far 79% (Fig.). The cumulative incidence for grade 2 and 3 infections in the first 90 days was 26.7%, where 2 patients died. One patient died at day 180 from liver biopsy complications for late onset hepatic sinusoidal obstruction syndrome. No surviving patient has had disease relapse.
It can be concluded that Orca-T is a safe and feasible transplant strategy in the RIC setting for patients with advanced hematological malignancies. Patients showed robust and early donor myeloid and eventual full donor T cell engraftment. The low incidence of acute and chronic GVHD with low rates of disease relapse, suggest retention of graft versus leukemia effect. Few studies of donor graft-engineered products have been successfully evaluated in the RIC setting and merits larger multicenter studies evaluating GVHD-free and RFS post-transplant to validate these findings.
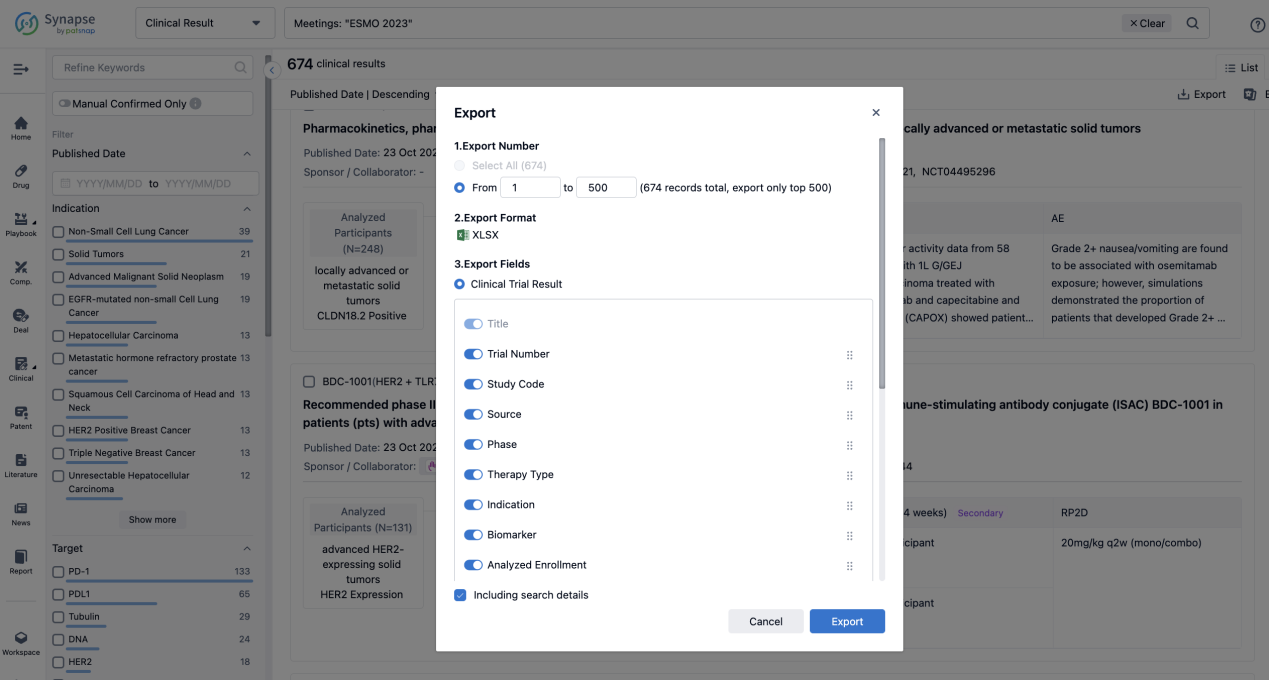
How to Easily View the Clinical Results Using Synapse Database?
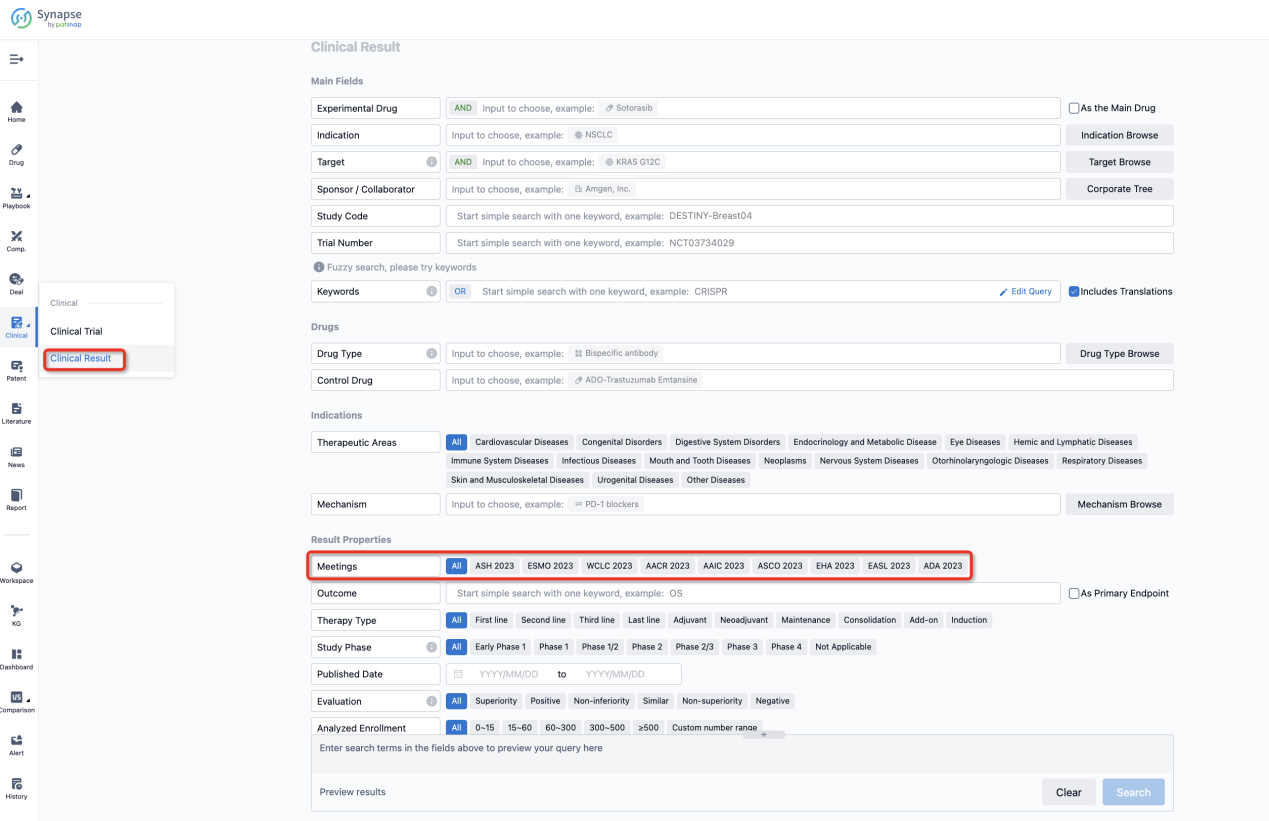
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
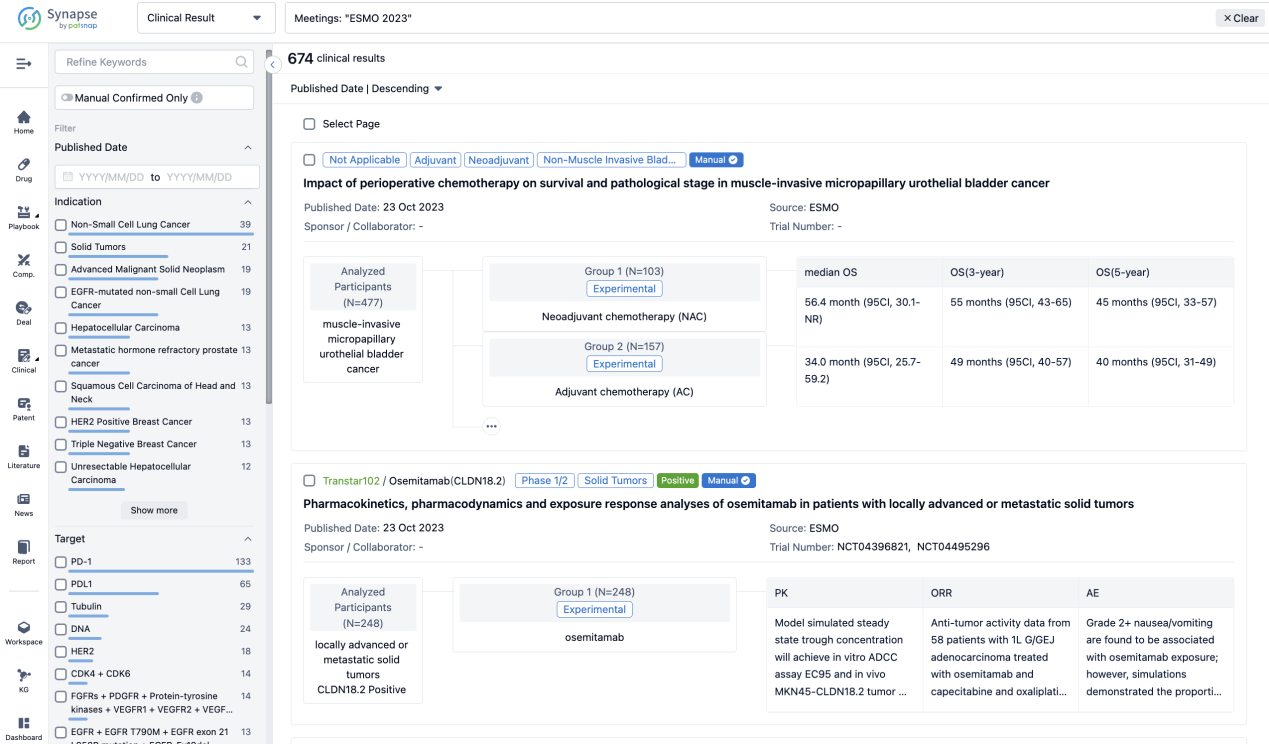
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
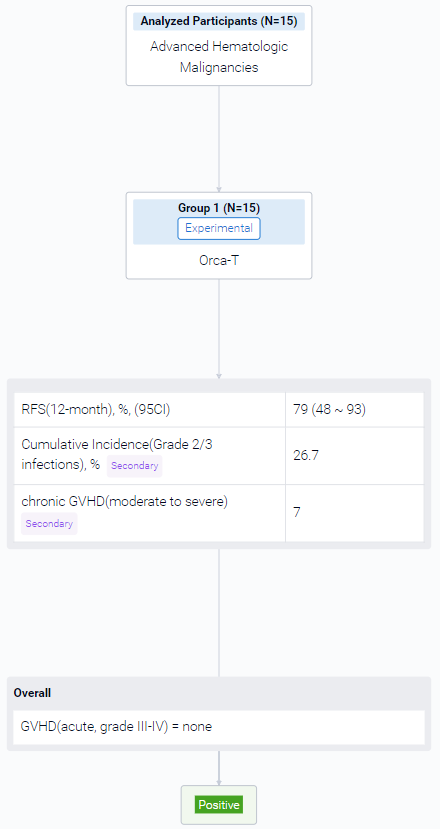
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!