Decoding Paroxetine Mesylate: A Comprehensive Study of its R&D Trends
Paroxetine Mesylate's R&D Progress
Paroxetine Mesylate is a small molecule drug that falls under the therapeutic area of “Other Diseases”. It specifically targets the serotonin transporter (SERT). The drug is primarily indicated for the treatment of vasomotor symptoms.
The originator organization of Paroxetine Mesylate is Synthon BV, a pharmaceutical company involved in the development and manufacturing of generic and innovative medicines.
Paroxetine Mesylate has reached the highest phase of development, which is approval. It received first approval in the United States in July 2003. This indicates that the drug has successfully completed the necessary clinical trials and regulatory processes to be deemed safe and effective for use in patients.
Vasomotor symptoms are a common occurrence in menopausal women and can include hot flashes and night sweats. Paroxetine Mesylate works by inhibiting the reuptake of serotonin, a neurotransmitter involved in regulating body temperature. By modulating serotonin levels, the drug helps to alleviate vasomotor symptoms and improve the quality of life for patients experiencing these symptoms.
The approval of Paroxetine Mesylate in 2003 marked an important milestone in the treatment of vasomotor symptoms. Prior to its approval, hormone replacement therapy (HRT) was the primary treatment option for menopausal symptoms. However, HRT has been associated with certain risks and side effects, leading to the need for alternative treatment options.
Paroxetine Mesylate provides a non-hormonal alternative for women experiencing vasomotor symptoms. Its approval in the United States demonstrates the safety and efficacy of this specific indication. Since its approval, Paroxetine Mesylate has likely been prescribed by healthcare professionals to patients with vasomotor symptoms, providing them with relief and improving their overall well-being.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Paroxetine Mesylate: SERT Inhibitors
From a biomedical perspective, serotonin transporter (SERT) inhibitors refer to a class of drugs that specifically target and inhibit SERT. The serotonin transporter is a protein responsible for the reuptake of serotonin in the brain, thereby regulating its levels and activity. By inhibiting SERT, these drugs prevent the reuptake of serotonin, leading to increased serotonin levels in the synaptic cleft between nerve cells. This increased serotonin concentration can have various effects on mood, cognition, and behavior.
SERT inhibitors are primarily used as antidepressant medications, as they help to alleviate symptoms of depression by enhancing serotonin neurotransmission. By blocking the reuptake of serotonin, SERT inhibitors prolong its activity, allowing it to bind to serotonin receptors for a longer duration. This ultimately leads to an overall increase in serotonin signaling, which is associated with improved mood and reduced depressive symptoms.
It's important to note that SERT inhibitors should only be used under medical supervision, as they can have potential side effects and interactions with other medications. Additionally, the specific mechanism of action and efficacy may vary depending on the particular SERT inhibitor being used.
Drug Target R&D Trends for Paroxetine Mesylate
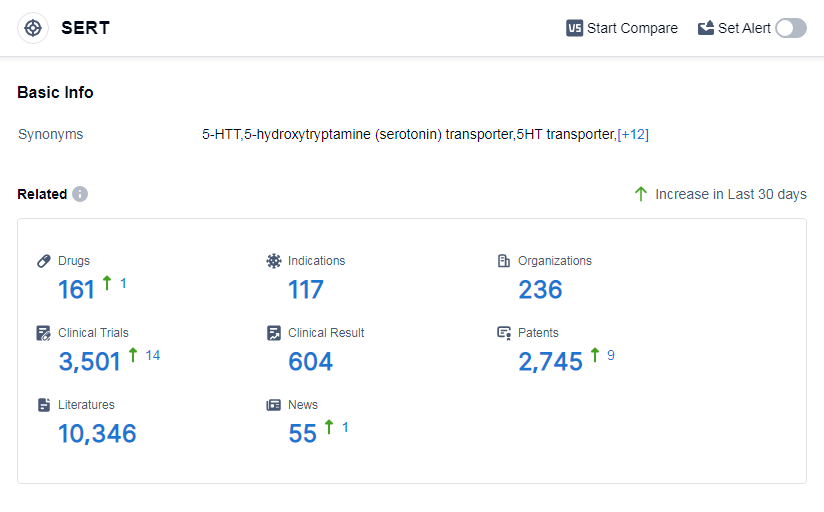
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 161 SERT drugs worldwide, from 236 organizations, covering 117 indications, and conducting 3501 clinical trials.
The analysis of the target SERT reveals a competitive landscape with multiple companies making progress in drug development. AbbVie, Inc., Viatris Inc., Pfizer Inc., Eli Lilly & Co., and Lundbeck Foundation are among the companies growing fastest under the target. The most common indications for approved drugs include depressive disorder, depressive disorder major, obsessive-compulsive disorder, panic disorder, and anxiety. Small molecule drugs and unknown drugs are progressing rapidly, indicating intense competition and ongoing research efforts. The United States, China, Japan, and the European Union are the leading countries orlocations in drug development for the target SERT. In particular,China has great progress. Overall, the target SERT presents opportunities for further research and development, especially in the areas of small molecule drugs and indications related to depressive disorders.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Paroxetine Mesylate is a small molecule drug developed by Synthon BV. It is indicated for the treatment of vasomotor symptoms and targets the serotonin transporter. The drug received its first approval in the United States in 2003 and has since provided an alternative treatment option for menopausal women experiencing vasomotor symptoms.





