Decoding primaquine phosphate: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Primaquine phosphate's R&D Progress
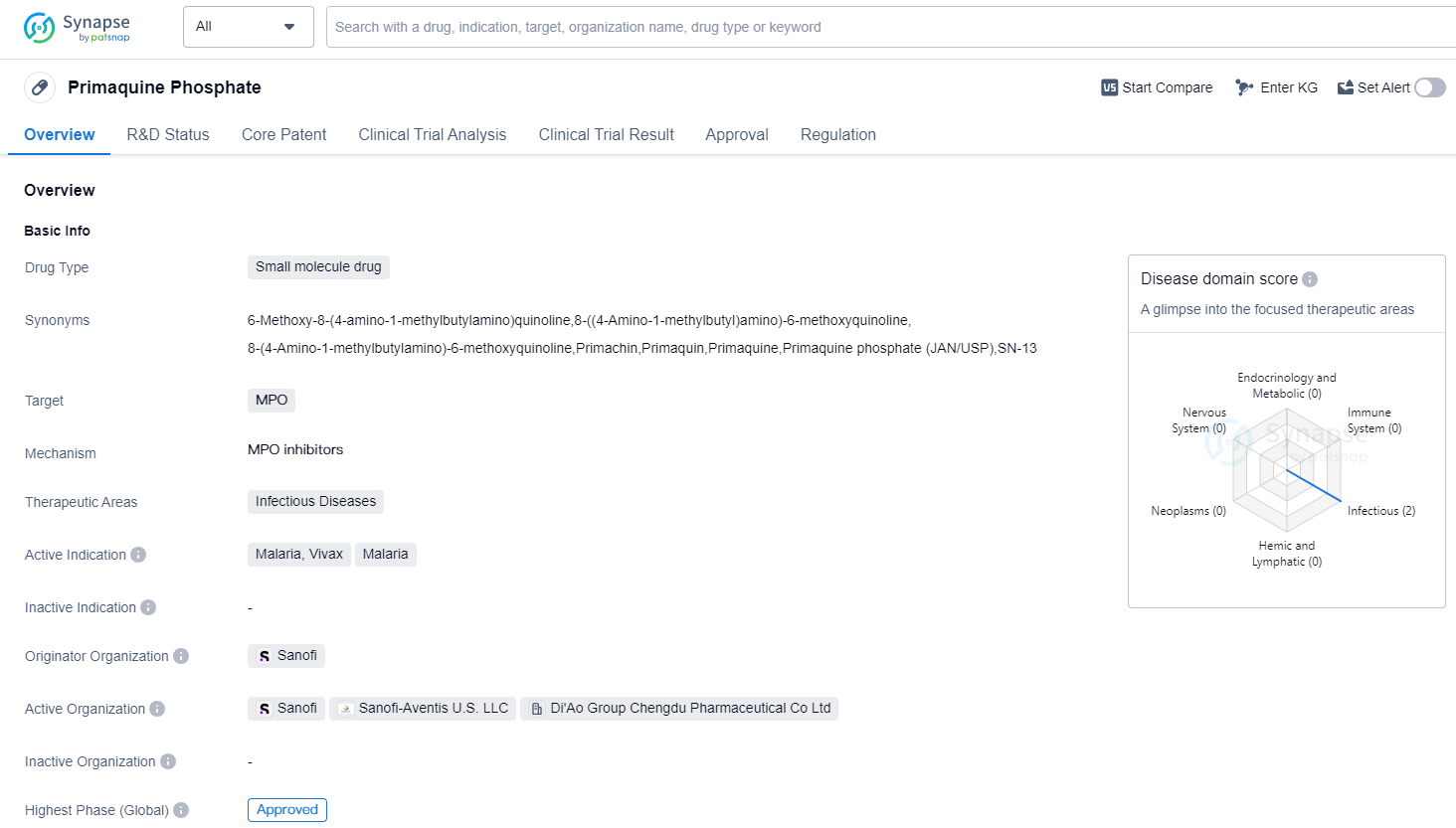
Primaquine Phosphate is a small molecule drug that falls under the therapeutic area of infectious diseases. It specifically targets MPO (myeloperoxidase), an enzyme involved in the immune response. The drug is primarily indicated for the treatment of malaria caused by the Plasmodium vivax parasite.
The originator organization of Primaquine Phosphate is Sanofi, a multinational pharmaceutical company. The drug has reached the highest phase of development which is approved globally. This indicates that it has successfully completed clinical trials and has been granted regulatory approval for use in patients.
Primaquine Phosphate was first approved globally in 1950, making it a well-established drug in the field of biomedicine. It has been used for several decades in the treatment of malaria, particularly the vivax form of the disease. The drug's long history of use suggests that it has a proven safety and efficacy profile.
In terms of regulation, Primaquine Phosphate is classified as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for primaquine phosphate: MPO inhibitors
MPO inhibitors are a type of medication that target and inhibit the activity of myeloperoxidase (MPO), an enzyme found in certain immune cells called neutrophils. MPO plays a role in the immune response by producing reactive oxygen species, which help to kill invading pathogens. However, excessive MPO activity can lead to oxidative stress and tissue damage in various inflammatory conditions.
From a biomedical perspective, MPO inhibitors are of interest in the field of biomedicine because they have the potential to modulate the immune response and reduce inflammation. By inhibiting MPO activity, these inhibitors can help mitigate the harmful effects of excessive oxidative stress and tissue damage associated with conditions such as chronic inflammation, cardiovascular diseases, and neurodegenerative disorders.
MPO inhibitors have been studied as potential therapeutic agents for various diseases, including atherosclerosis, rheumatoid arthritis, and chronic obstructive pulmonary disease (COPD). By targeting MPO, these inhibitors aim to reduce the production of reactive oxygen species and limit inflammation, thereby potentially improving patient outcomes.
It is important to note that MPO inhibitors are still under investigation, and their clinical utility and safety profiles are being evaluated through preclinical and clinical studies.
Drug Target R&D Trends for primaquine phosphate
MPO, or myeloperoxidase, is an enzyme found in the human body that plays a crucial role in the immune system. It is primarily produced by white blood cells called neutrophils and is involved in the process of killing microorganisms, such as bacteria and fungi. MPO catalyzes the production of hypochlorous acid, a powerful antimicrobial agent, which helps in the destruction of pathogens. Additionally, MPO is also implicated in various inflammatory diseases and has been studied as a potential biomarker for cardiovascular diseases. Understanding the role of MPO in the human body is essential for developing therapeutic interventions and diagnostic tools in the pharmaceutical industry.
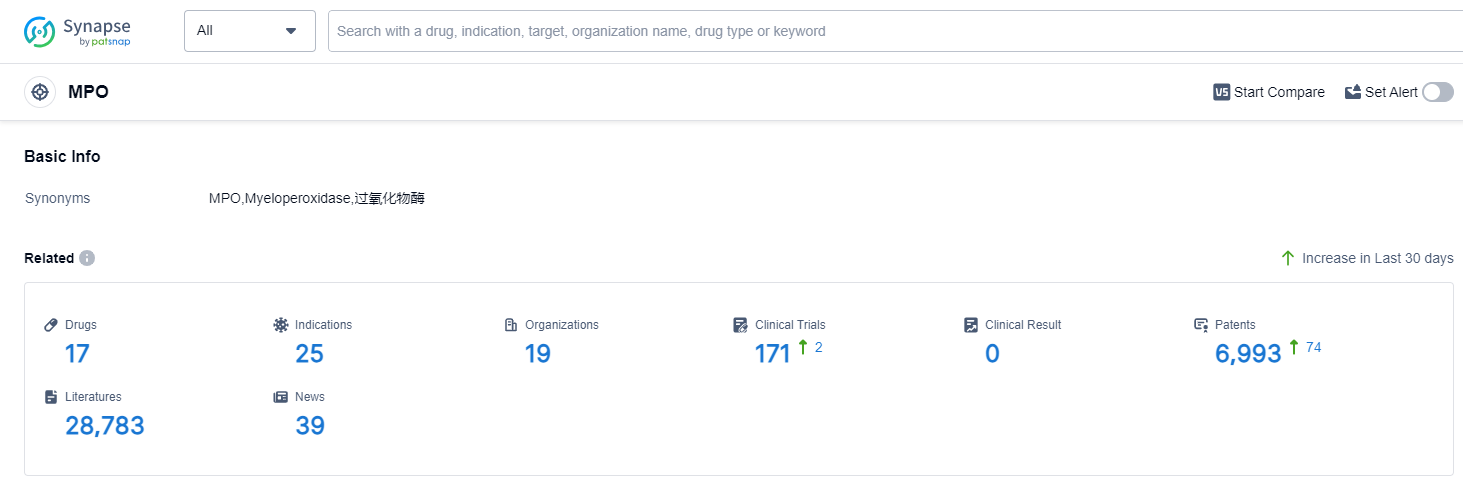
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 17 MPO drugs worldwide, from 19 organizations, covering 25 indications, and conducting 171 clinical trials.
Overall, the target MPO presents a competitive landscape with a focus on diverse indications, drug types, and global development efforts. The future development of the target MPO is promising, with ongoing research and development activities by various companies and countries.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Primaquine Phosphate is a small molecule drug developed by Sanofi for the treatment of malaria, specifically the vivax form. It targets the MPO enzyme and has been approved for use globally since 1950. The drug is classified as an orphan drug, indicating its potential to address unmet medical needs in the field of infectious diseases.