Deep Scientific Insights on teprotumumab-trbw's R&D Progress, Mechanism of Action, and Drug Target
Teprotumumab-trbw's R&D Progress
Teprotumumab-TRBW is a monoclonal antibody drug that targets the IGF-1R (Insulin-like Growth Factor-1 Receptor). It has been developed by Genmab A/S and has received approval for use in the treatment of Graves Ophthalmopathy and Scleroderma, Diffuse. The drug falls under the therapeutic areas of Skin and Musculoskeletal Diseases, Immune System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, and Eye Diseases.
Teprotumumab-TRBW received its first approval in the United States in January 2020. The drug has undergone rigorous testing and has reached the highest phase of development, which is the approved stage. This indicates that it has successfully completed clinical trials and has met the necessary regulatory requirements for safety and efficacy.
In terms of regulatory status, Teprotumumab-TRBW has been granted several designations, including Fast Track, Orphan Drug, and Breakthrough Therapy. These designations are given to drugs that show potential in treating serious or life-threatening conditions and aim to expedite their development and approval process.
Graves Ophthalmopathy is a condition characterized by inflammation and swelling of the tissues around the eyes, often associated with an overactive thyroid gland. Scleroderma, Diffuse, on the other hand, is a chronic autoimmune disease that affects the skin and connective tissues. Teprotumumab-TRBW has shown promise in treating both of these conditions.
As a monoclonal antibody, Teprotumumab-TRBW works by targeting the IGF-1R, which plays a role in cell growth and survival. By inhibiting this receptor, the drug aims to reduce inflammation and improve symptoms associated with Graves Ophthalmopathy and Scleroderma, Diffuse.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for teprotumumab-trbw: IGF-1R antagonists
IGF-1R antagonists are a type of drugs that target the insulin-like growth factor 1 receptor (IGF-1R). The IGF-1R is a cell surface receptor that plays a crucial role in cell growth, proliferation, and survival. It is involved in various biological processes, including embryonic development, tissue repair, and metabolism.
From a biomedical perspective, IGF-1R antagonists are designed to inhibit the activity of the IGF-1R by blocking its binding sites or interfering with downstream signaling pathways. By doing so, these antagonists can prevent the binding of insulin-like growth factors (IGFs) to the receptor and inhibit the activation of cellular processes associated with IGF-1R signaling.
IGF-1R antagonists have shown potential therapeutic applications in cancer treatment. Overexpression or dysregulation of IGF-1R has been observed in various types of cancer, and it is associated with tumor growth, metastasis, and resistance to chemotherapy. By targeting IGF-1R, these antagonists can potentially inhibit tumor cell proliferation, induce apoptosis (programmed cell death), and enhance the effectiveness of other cancer therapies.
It is important to note that while IGF-1R antagonists hold promise as potential cancer therapeutics, further research and clinical trials are needed to fully understand their efficacy, safety profile, and optimal usage in different cancer types.
Drug Target R&D Trends for teprotumumab-trbw
IGF-1R, or insulin-like growth factor 1 receptor, plays a crucial role in the human body. It is a cell surface receptor that binds to insulin-like growth factors (IGFs) and regulates cell growth, proliferation, and survival. IGF-1R activation triggers a cascade of signaling pathways that promote cell division, protein synthesis, and tissue development. It is involved in various physiological processes, including bone growth, muscle development, and tissue repair. Dysregulation of IGF-1R signaling has been implicated in several diseases, including cancer, diabetes, and neurodegenerative disorders. Understanding the role of IGF-1R is essential for developing targeted therapies and interventions in the pharmaceutical industry.
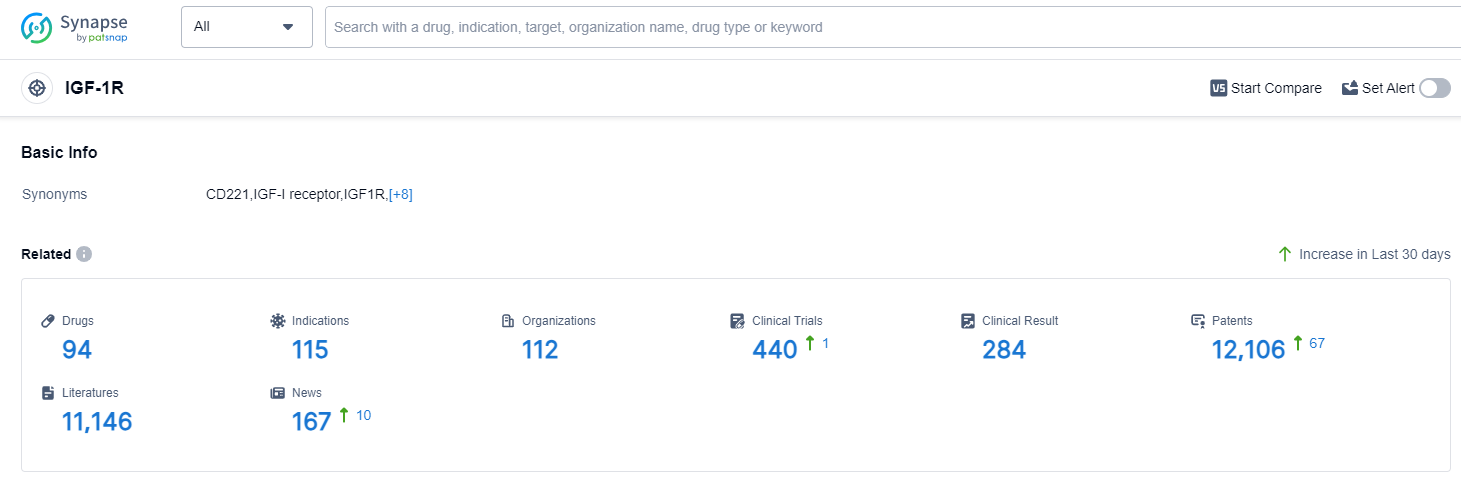
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 94 IGF-1R drugs worldwide, from 112 organizations, covering 115 indications, and conducting 440 clinical trials.
The analysis of target IGF-1R reveals a competitive landscape with several companies showing significant growth and R&D progress. Novartis AG, Takeda Pharmaceutical Co., Ltd., Horizon Therapeutics Plc, and Astellas Pharma, Inc. are among the fastest-growing companies. Approved indications for drugs targeting IGF-1R cover a wide range of diseases, indicating the potential of these drugs in various therapeutic areas. The most rapidly progressing drug types include growth factors, small molecule drugs, monoclonal antibodies, and biosimilars. The United States, European Union, and Japan are leading in terms of drug development, with China also showing progress. Further analysis of research institutions and progress in China is recommended. Overall, the target IGF-1R presents a promising landscape for future development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Teprotumumab-TRBW is an approved monoclonal antibody drug that targets the IGF-1R and has shown efficacy in treating Graves Ophthalmopathy and Scleroderma, Diffuse. Its approval in the United States and the regulatory designations it has received highlight its potential as a therapeutic option for patients with these conditions.