Early data has been released by Inhibrx about INBRX-109's Phase 1 trial, focused on Ewing Sarcoma treatment
Inhibrx, Inc., a biopharmaceutical firm in the clinical stage that focuses on formulating treatments for cancer and rare conditions, declared the initial results concerning efficacy and safety from the Phase 1 trial. This trial was for INBRX-109, used in conjunction with Irinotecan and Temozolomide, designed for treating advanced or metastatic, non-removable Ewing sarcoma.
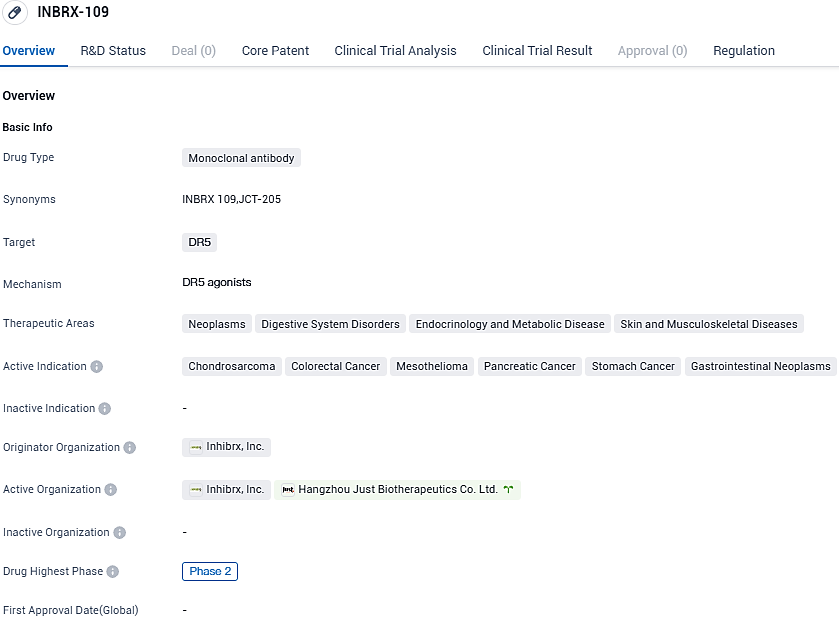
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Out of the 13 assessable patients including 7 with classical Ewing sarcoma and 6 with round cell sarcoma, a disease control rate of 76.9% was recorded, signifying 10 out of 13 patients, as evaluated by RECISTv1.1. A durable clinical advantage was noted in 4 people (30.8%) who demonstrated disease control for more than 6 months. As of September 8, 2023, the longest period of steady disease was over 10 months and ongoing and 7 of the 13 remained part of the study.
INBRX-109 is a tetravalent death receptor 5 (DR5) agonist antibody which has been precision-engineered to leverage the tumor-inclined cell death brought about by DR5 activation. Overall, the combination of INBRX-109 with IRI/TMZ was well tolerated. Diarrhea, nausea, and fatigue were the most frequent unfavorable events, all aligning with the known safety characteristics of IRI/TMZ. No liver-related incidents of grade 3 or superior occurred.
"I am optimistic about the preliminary data in relapsed/refractory Ewing sarcoma patients. This group of patients have a high unfulfilled need and limited efficient therapy options," expressed Dr. Rashmi Chugh, MD, a Professor of Internal Medicine at the Division of Hematology/Oncology at the University of Michigan Rogel Comprehensive Cancer Center. "I anticipate the continued recruitment in this group of patients and am eager to see the outcomes of the enlargement."
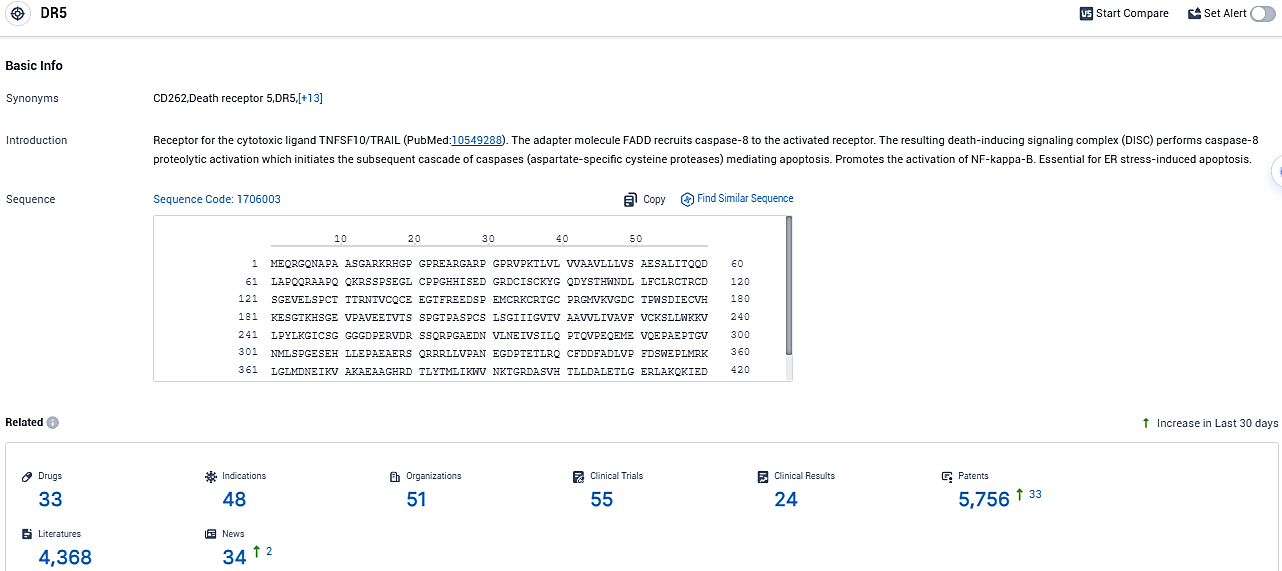
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 9, 2023, there are 33 investigational drugs for the DR5 target, including 48 indications, 51 R&D institutions involved, with related clinical trials reaching 55, and as many as 4368 patents.
In June 2021, Inhibrx initiated a randomized, blinded, placebo-controlled, registration-enabling Phase 2 trial of INBRX-109 in conventional chondrosarcoma, which is currently ongoing. In January 2021, the FDA granted Fast Track designation to INBRX-109 for the treatment of patients with unresectable or metastatic conventional chondrosarcoma and orphan-drug designation to INBRX-109 for chondrosarcoma in the United States.