EC Grants Marketing Approval for EirGenix's Biosimilar for Breast Cancer
EirGenix Inc. has delivered news regarding the receipt of a marketing endorsement from the European Commission for their trastuzumab biosimilar medication (EG12014). This product is prepped for commercialization by their business collaborator, Sandoz.
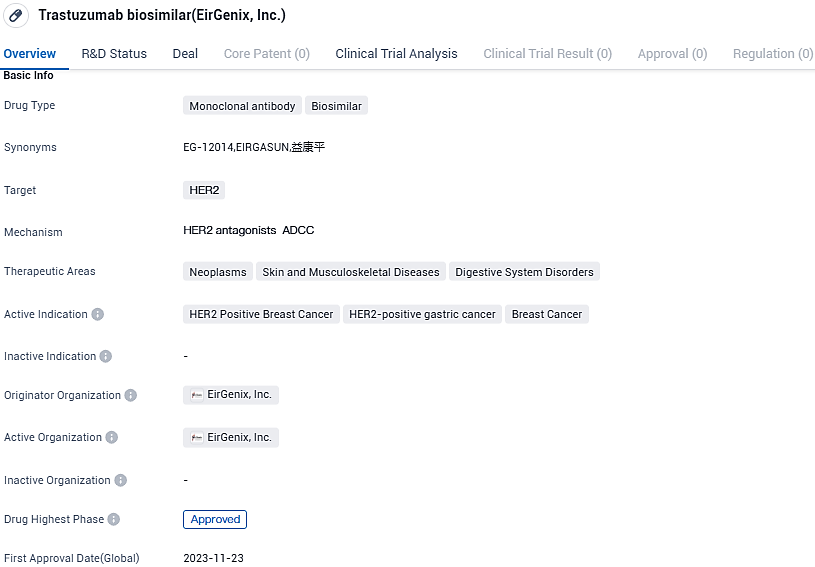
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The EU's marketing authorization will be applicable to the management of human epidermal growth factor receptor 2 positive (HER2-positive) breast cancer and metastatic stomach cancers. This is the same as the approved indications by the EC for the reference biologic, Herceptin®.
Sandoz AG and EirGenix entered into a licensing contract in April 2019. As per this contract, EirGenix Inc. will continue to shoulder the development and production of trastuzumab, while Sandoz will acquire the rights to market the medicine globally upon approval. Breast and stomach cancers rank among the most frequently reported in Europe, and in combination, they contribute to nearly 200,000 deaths every year. Biosimilars have a huge potential to boost cancer care by significantly enhancing access to crucial medicaments.
Every year, more than 355,000 women are diagnosed with breast cancer, and with 92,000 annual fatalities, it is the leading cause of cancer mortality among females. Stomach cancer ranks sixth in prevalence among all cancer types, and with 107,000 deaths every year, it is the fourth leading cause of cancer-related deaths in Europe. In about 20% of breast cancer and 30% of stomach cancer cases identified, an overexpression of HER2 protein is noticed which leads to uncontrolled cell growth and division.
HER2 cancers are extremely aggressive forms of cancer that react positively to targeted therapy. The overall European authorization of EG12014 enlarges the access to a vital, high-quality cure for breast and stomach cancers, thus reducing the disease burden on patients and producing meaningful savings for healthcare systems for longer-term sustainability.
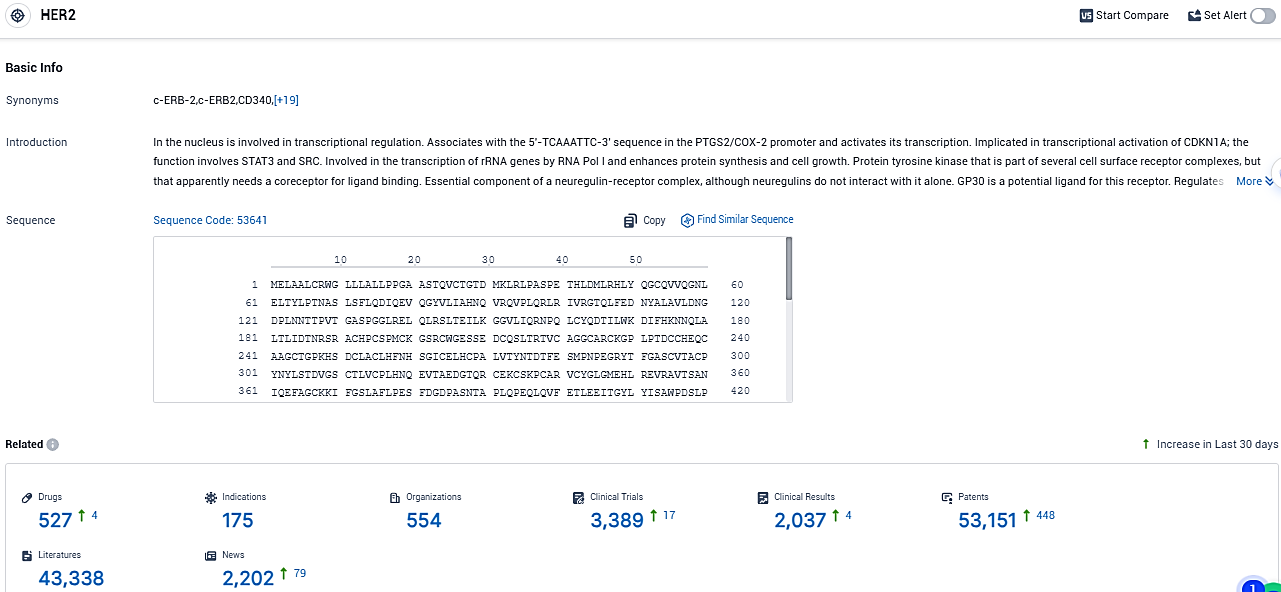
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 30, 2023, there are 527 investigational drugs for the HER2 target, including 175 indications, 554 R&D institutions involved, with related clinical trials reaching 3389, and as many as 53151 patents.
EirGenix's trastuzumab biosimilar drug (EG12014) also received market approval by TFDA in June. The application of the health insurance pricing was approved by the National Health Insurance Administration under the Ministry of Health and Welfare in mid-September, paving the way for the formal launch of the product in Taiwan.