Eisai Submits Biologics License Application to US FDA for LEQEMBI® (lecanemab-irmb) Alzheimer's Treatment, Gains Fast Track Status
Eisai Co., Ltd. and Biogen Inc. revealed that Eisai has started the rolling submission of a Biologics License Application to the U.S. Food and Drug Administration for the lecanemab-irmb subcutaneous autoinjector intended for weekly maintenance dosing. This follows the FDA's Fast Track designation. LEQEMBI is used to treat Alzheimer's disease in patients who are in the Mild Cognitive Impairment or mild dementia stage.
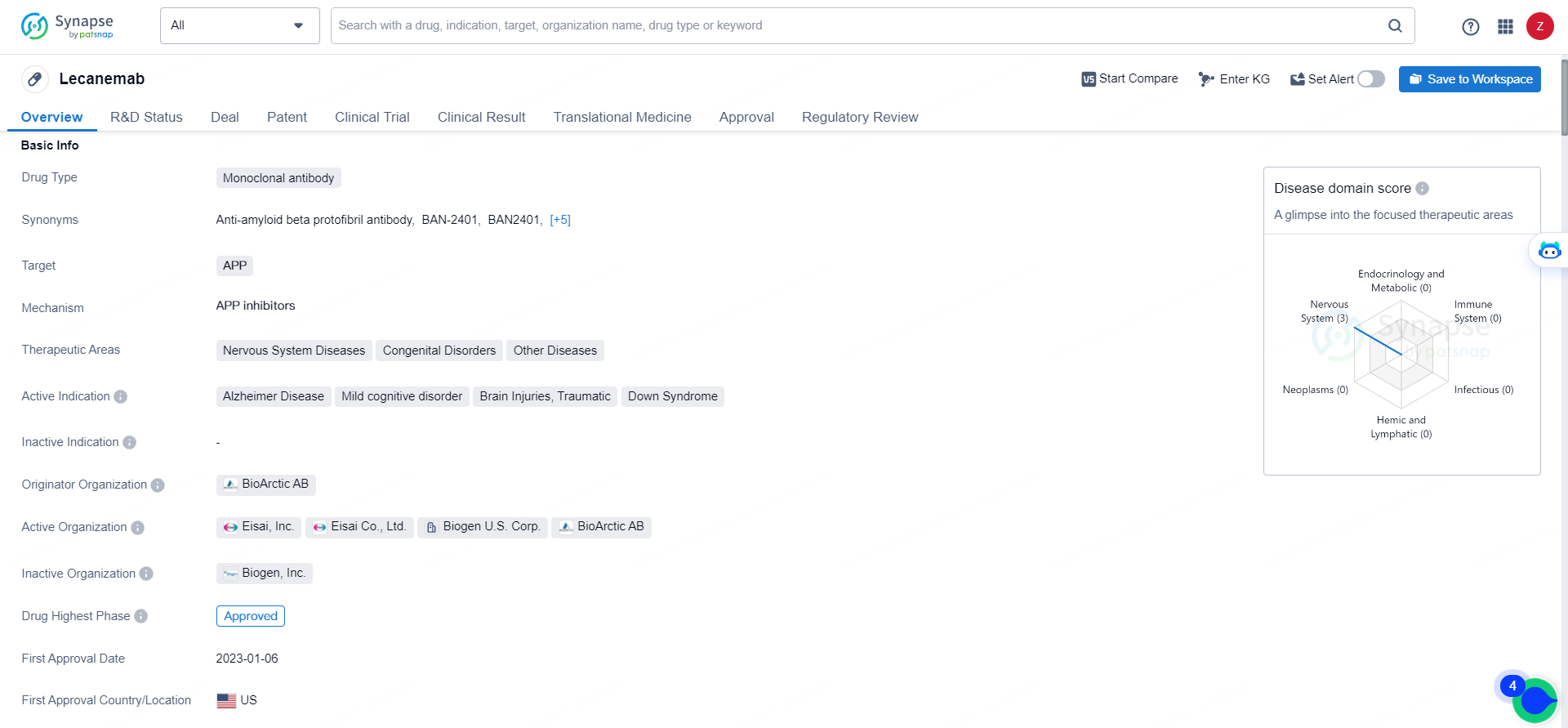
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Biologics License Application (BLA) leverages data from the Clarity AD open-label extension and modeling based on observed data. Pending FDA approval, the LEQEMBI autoinjector may facilitate home or clinical administration. The injection process is more time-efficient compared to the IV formulation.
Under review is the subcutaneous autoinjector's 360 mg weekly maintenance regimen, where patients transitioning from the biweekly IV initiation phase would receive weekly doses to maintain effective drug levels, supporting the clearance of highly toxic protofibrils that can persist in causing neuronal damage even post-clearance of amyloid-beta plaques from the brain.
Alzheimer's disease (AD) represents a continuous neurotoxic process that starts before and extends beyond plaque deposition. Evidence suggests that early and sustained treatment may extend benefits even after plaque removal from the brain.
This subcutaneous autoinjector offers ease of use for patients and caregivers, potentially lessening hospital and nursing care requirements compared to intravenous administration. Besides potentially sustaining clinical and biomarker benefits, subcutaneous maintenance may offer greater convenience for ongoing patient treatment and caregiver involvement.
LEQEMBI has been approved in the U.S., Japan, and China, with applications under review in the European Union, Australia, Brazil, Canada, Hong Kong, Great Britain, India, Israel, Russia, Saudi Arabia, South Korea, Taiwan, Singapore, and Switzerland. In March 2024, Eisai submitted a Supplemental Biologics License Application to the FDA for monthly LEQEMBI intravenous maintenance dosing.
Eisai leads the global development and regulatory submissions for lecanemab, with both companies co-commercializing and co-promoting the product, while Eisai retains final decision-making authority. Lecanemab, a monoclonal antibody developed by BioArctic AB, targets the amyloid precursor protein (APP) and is designated for treating Alzheimer's disease, mild cognitive impairment, traumatic brain injuries, and Down syndrome.
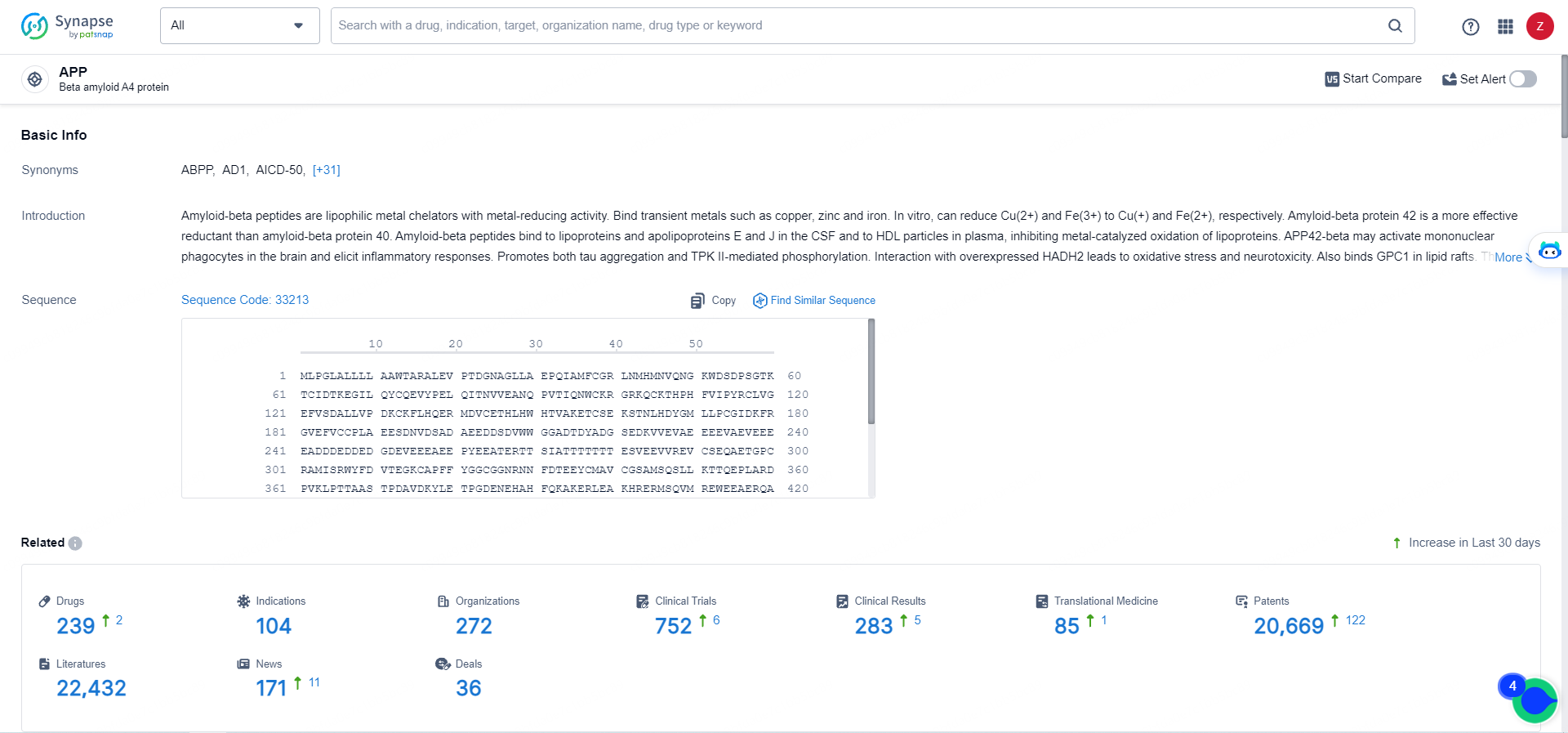
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 16, 2024, there are 239 investigational drugs for the APP targets, including 104 indications, 272 R&D institutions involved, with related clinical trials reaching 752, and as many as 20669 patents.
Lecanemab's approval and regulatory designations highlight its potential to make a meaningful impact on the treatment of Alzheimer's disease, mild cognitive disorder, traumatic brain injuries, and Down syndrome. Its development represents a significant advancement in the pharmaceutical industry, offering hope for patients and healthcare providers seeking effective treatments for these challenging conditions.