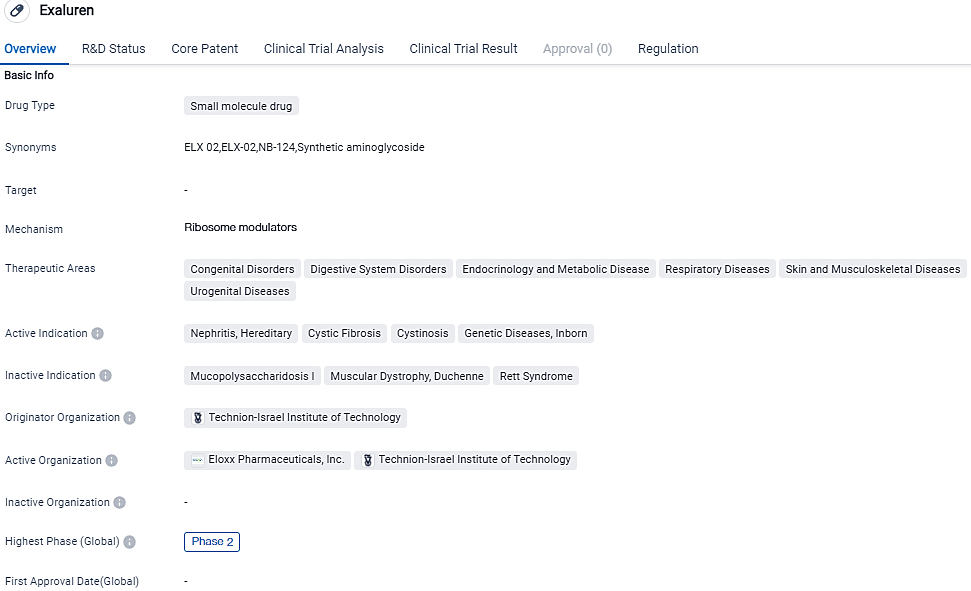
Eloxx Pharmaceuticals Shares Program Report on Phase 2 Alport Trial involving ELX-02
Eloxx Pharmaceuticals, Inc., a pioneer in ribosomal RNA-focused genetic treatments for rare diseases, provided new information on the development of ELX-02 in the context of handling Alport syndrome caused by nonsense mutations. This update includes further encouraging outcomes from the firm's Phase 2 clinical research which has been assessing ELX-02.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Alport syndrome, a rare hereditary kidney disease caused by alterations in the COL4A3/4/5 genes, leads to podocyte damage and impaired renal filtration function, resulting in proteinuria.
Sumit Aggarwal, Eloxx's President and CEO, noted, "Key opinion leaders and significant members of the Alport syndrome community uniformly and ardently support the potential of ELX-02 to address this grievous condition, as well as the urgency to advance to a landmark study, as indicated by our Phase 2 study findings."
Encouraged by the current clinical findings, Eloxx has presented an IND application to the U.S. FDA for the use of ELX-02 in the treatment of Alport syndrome with nonsense mutations. The approval of the IND application will enable the participation of U.S. based sites in the forthcoming crucial trial.
In the Phase 2 open-label clinical trial, the three patients who received ELX-02 exhibited an enhancement in the effacement of podocyte foot processes, a characteristic feature of proteinuric kidney diseases such as Alport syndrome. Electron microscopic images of biopsy samples demonstrated an enhancement in the glomerular basement membrane and re-attachment of podocyte foot processes in all treated patients.
This data substantiates the disease-altering effect and protein restoration potential of ELX-02, as well as the potential for improvement in proteinuria with a more extended treatment duration. Podocytes are specialized cells that adhere to the glomerular basement membrane and sprout finger-like extensions known as foot processes that facilitate efficient ultrafiltration. Podocyte damage triggers the effacement of podocyte foot processes and proteinuria in nearly every case of Alport syndrome.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
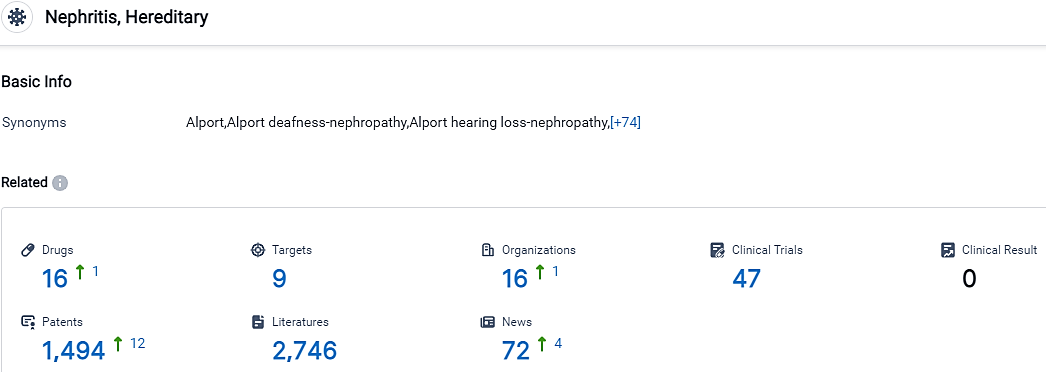
According to the data provided by the Synapse Database, As of September 13, 2023, there are 16 investigational drugs for the Alport syndrome, including 9 targets, 16 R&D institutions involved, with related clinical trials reaching 47,and as many as 1494 patents.
Reata Pharmaceuticals, Inc. and Kirin Holdings Co., Ltd. are the companies with the highest development phase under the Alport syndrome, Hereditary. The target analysis reveals multiple potential therapeutic targets, including NF-κB + Nrf2, AT1R + ETA, FXR, miR-21, ETA, ABCA1, BDNF + VDCCs, MMP12, and INSR. Overall, the indication Nephritis, Hereditary presents a competitive landscape with diverse R&D efforts and potential for future development.