EMA Approves Bio-Thera's BAT1706: A Bevacizumab Biosimilar to Avastin®
Bio-Thera Solutions Inc., an established biopharmaceutical company engaged in the development of new therapies and biosimilars, announced that BAT1706 (bevacizumab), a biosimilar to Avastin, has been approved by the EMA.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
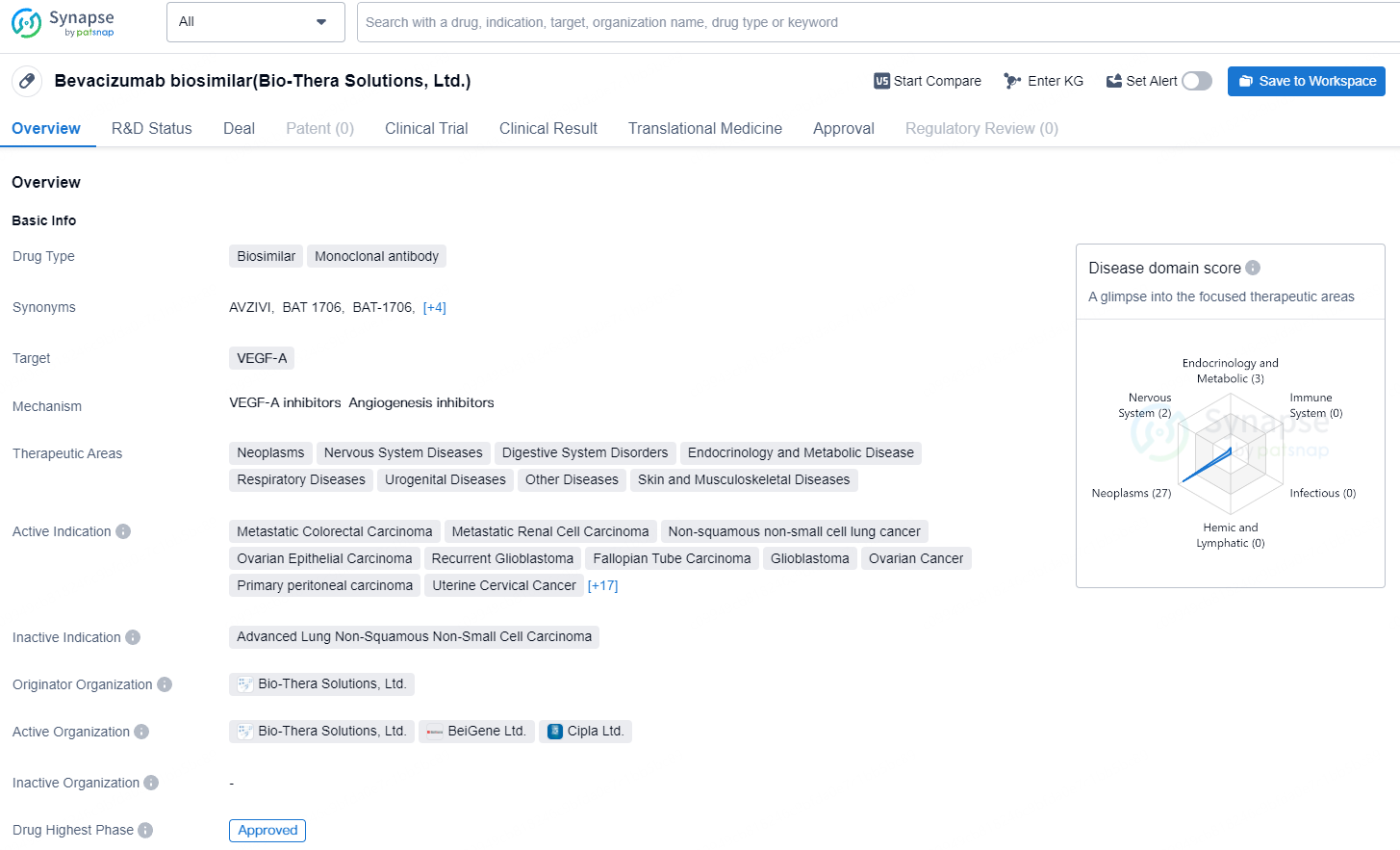 Sandoz AG and its affiliates hold the rights to commercialize BAT1706 in Europe under the brand name Avzivi. BAT1706 represents Bio-Thera’s second product sanctioned by the EMA, and it is also the company’s second product to gain marketing authorization from the NMPA, US FDA, and EMA.
Sandoz AG and its affiliates hold the rights to commercialize BAT1706 in Europe under the brand name Avzivi. BAT1706 represents Bio-Thera’s second product sanctioned by the EMA, and it is also the company’s second product to gain marketing authorization from the NMPA, US FDA, and EMA.
“The EMA approval of BAT1706 stands as another critical milestone for Bio-Thera, denoting our second EMA-endorsed product,” stated Shengfeng Li, CEO of Bio-Thera. “With our biosimilar pipeline continuing to evolve, we aim to achieve more biosimilar approvals, thereby broadening patient access to essential therapies.”
Bio-Thera and Sandoz AG formalized a license and commercialization deal for BAT1706 in September 2021. According to the terms of this arrangement, Bio-Thera is in charge of the product’s development and manufacturing, while Sandoz handles its commercialization in the European Union, the United States, and other global markets.
BAT1706 (Avzivi, bevacizumab) is a humanized monoclonal antibody targeting VEGF. It binds specifically to VEGF and inhibits the interaction of VEGF with its receptor, which reduces neovascularization and promotes the degradation of existing blood vessels, thereby curbing tumor growth.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
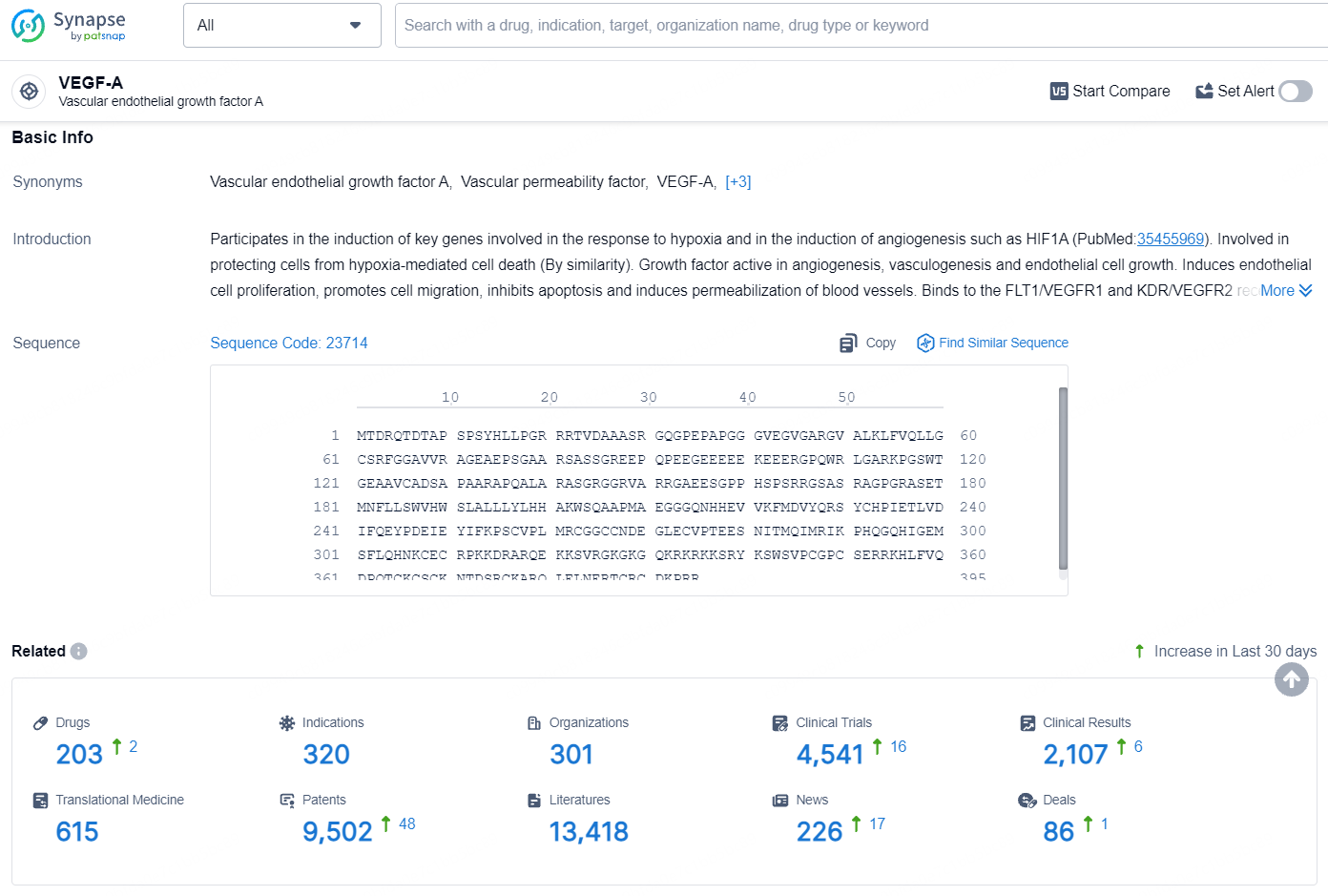
According to the data provided by the Synapse Database, As of August 5, 2024, there are 203 investigational drugs for the VEGF-A target, including 320 indications, 301 R&D institutions involved, with related clinical trials reaching 4541, and as many as 9502 patents.
BAT1706 is a biosimilar drug categorized as a monoclonal antibody which targets VEGF-A. Bio-Thera Solutions, Ltd. is the originator organization of this drug, and it has received approval for usage in all its therapeutic areas, both globally and within China. The first approval for global use of the Bevacizumab biosimilar was granted in November 2021 in China.