Ensho Therapeutics Debuts with Oral α4β7 Inhibitor for Phase 2 Trials in IBD
Ensho Therapeutics, Inc., a private clinical-stage biopharmaceutical company specializing in creating innovative oral treatments for inflammatory diseases, announced its launch. The company revealed that it has secured a portfolio of oral α4β7 integrin inhibitors under an exclusive global license agreement with EA Pharma Co., Ltd., a division of Eisai Co., Ltd. This agreement grants Ensho the rights to develop, produce, and market these assets worldwide, excluding specific regions in Asia.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
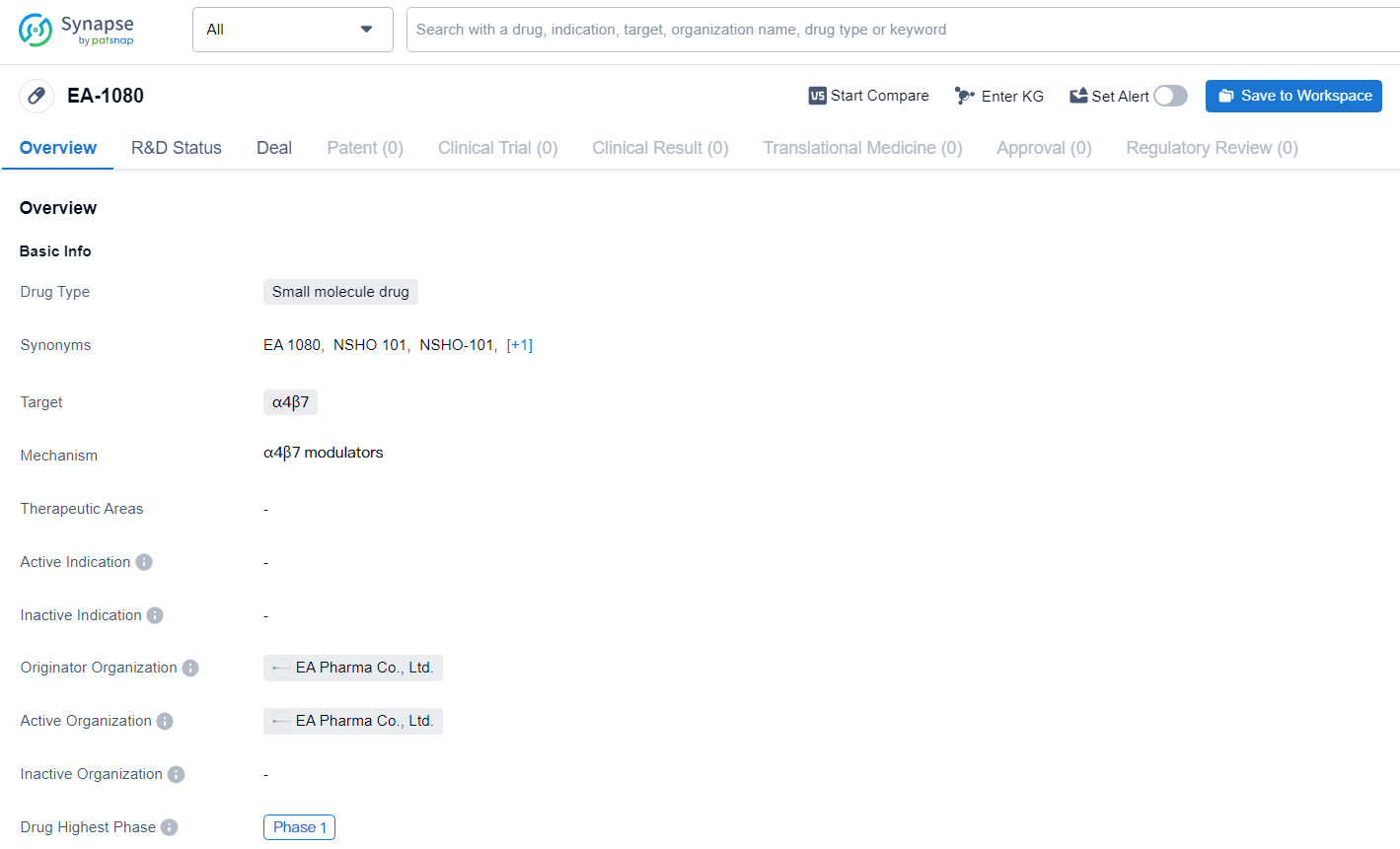
Building on the findings from an extensive Phase 1 clinical study, NSHO-101, (also known as EA1080), as its primary drug candidate. The Phase 1 study assessed both single and multiple ascending doses of NSHO-101 in more than 180 healthy participants. The results indicated favorable pharmacokinetics and pharmacodynamics, including effective target engagement. Moreover, NSHO-101 was found to be generally safe and well-tolerated. These results support Ensho’s plan to commence Phase 2 clinical trials in the first half of 2025, beginning with ulcerative colitis.
Neena Bitritto-Garg, CFA, founder, president, and CEO of Ensho, said, “We are excited to acquire this portfolio of oral selective α4β7 integrin inhibitors, which originated from AJM300, an oral α4 inhibitor approved in Japan for induction therapy in UC. My previous experience with Eisai and EA Pharma's commitment to continual improvement in drug development inspired me to establish Ensho around this portfolio. We believe NSHO-101 could be a groundbreaking alternative therapy for patients enduring IBD despite multiple treatments.”
Ensho has assembled a team of top-tier executives who have decades of experience in the field of inflammatory and gastrointestinal disease drug development. This team will collaborate with Ms. Bitritto-Garg to develop and potentially market these significant oral agents for the inflammatory bowel disease market.
Ms. Bitritto-Garg added, “I am delighted to welcome our experienced executive team to Ensho, including Bittoo Kanwar, M.D., the former chief medical officer of Telavant, which was acquired by Roche last year. With the team’s extensive background in IBD drug development, we are poised to move swiftly and strategically into Phase 2 clinical development with NSHO-101.”
Bittoo Kanwar, M.D., remarked, “Joining Neena to advance Ensho and its portfolio of promising IBD treatments was an incredibly appealing opportunity, given the substantial unmet medical needs in IBD, the verified mechanism of α4β7, and the potential of our innovative, oral clinical candidates to offer unique benefits to patients and healthcare providers looking for new therapeutic options. In partnership with our newly established Ensho team, many of whom have a long history of collaboration, we eagerly anticipate advancing our lead asset, NSHO-101, into Phase 2 development early next year.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
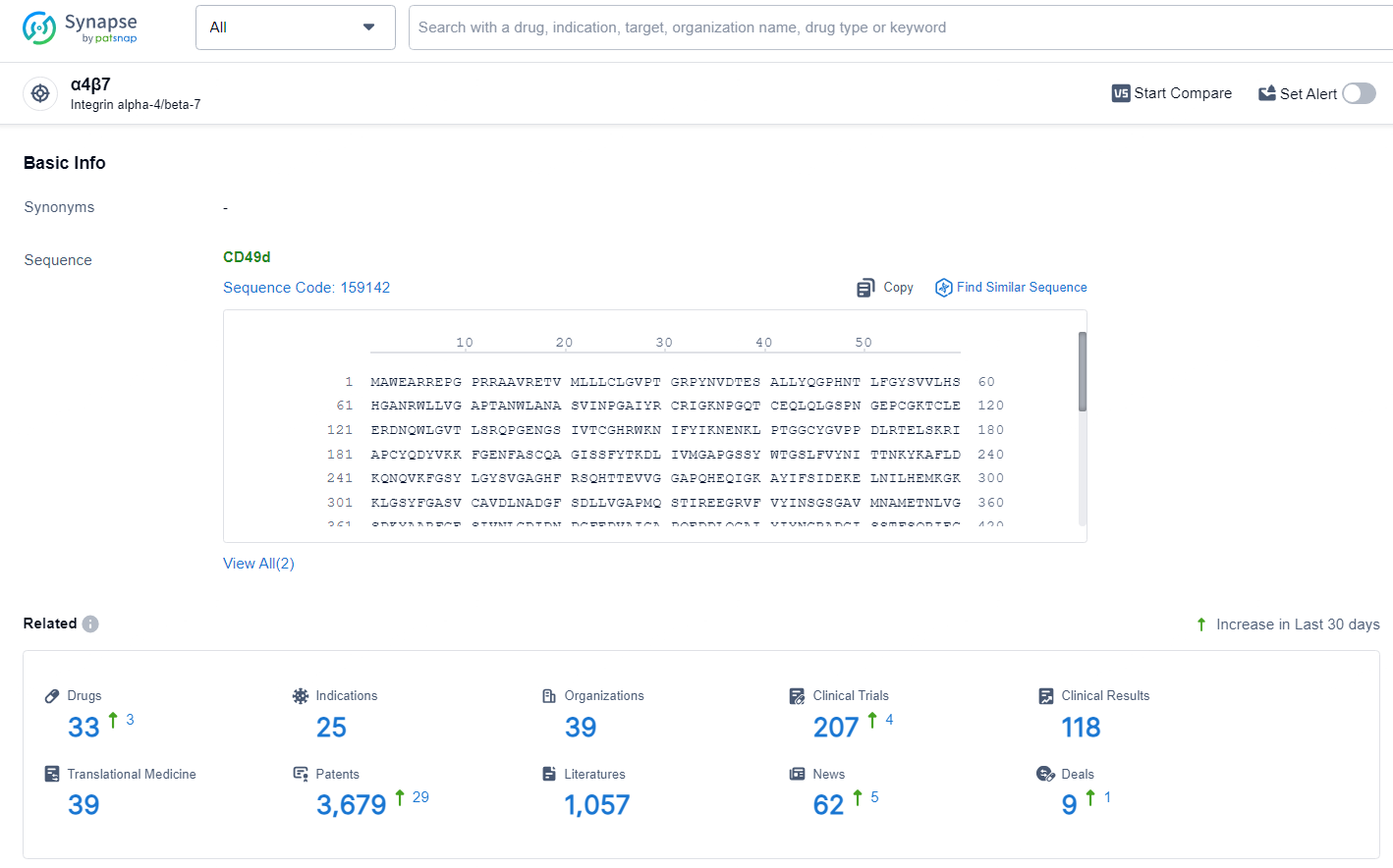
According to the data provided by the Synapse Database, As of July 3, 2024, there are 33 investigational drugs for the α4β7 target, including 25 indications, 39 R&D institutions involved, with related clinical trials reaching 207, and as many as 3679 patents.
EA-1080 is currently in Phase 1 of global development, indicating that it is undergoing initial clinical trials to evaluate its safety and potential efficacy in human subjects. As the development of EA-1080 progresses, further clinical data will be needed to determine its potential as a therapeutic option in the field of biomedicine.