Epalrestat Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Epalrestat's R&D Progress
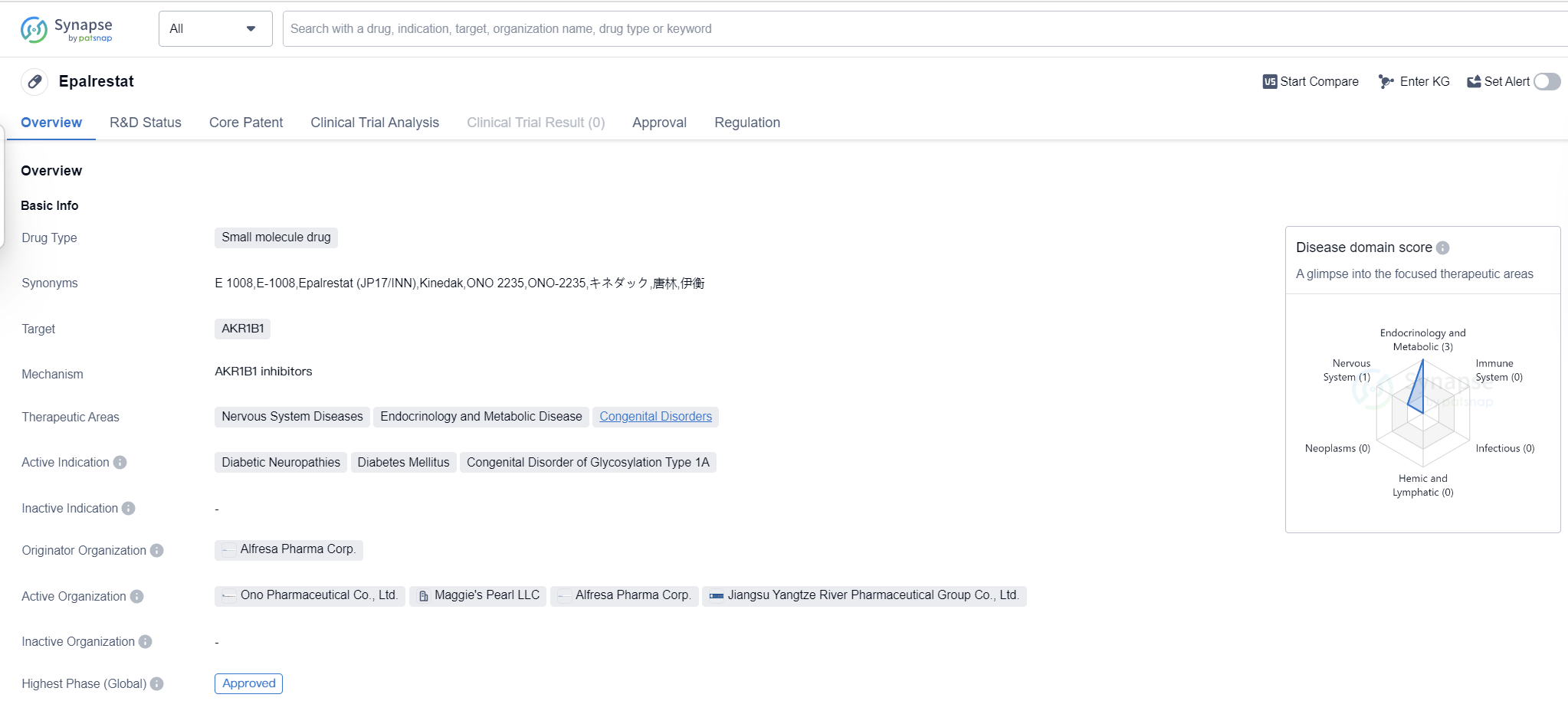
Epalrestat is a small molecule drug that targets AKR1B1 and is used in the treatment of various conditions related to the nervous system, endocrinology and metabolic diseases, as well as congenital disorders. Its active indications include diabetic neuropathies, diabetes mellitus, and congenital disorder of glycosylation type 1A.
The drug was developed by Alfresa Pharma Corp., a pharmaceutical organization based in Japan. Epalrestat received its first approval in May 1992 in Japan, making it the first country/location where the drug was authorized for use. It is important to note that Epalrestat is classified as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of patients.
Epalrestat has reached the highest phase of development which is approved globally. This indicates that the drug has successfully completed clinical trials and has been deemed safe and effective for use in patients. The fact that it has obtained regulatory approval in multiple countries suggests that it has met the necessary standards and requirements for pharmaceutical products.
As a small molecule drug, Epalrestat is likely to have a specific molecular structure that allows it to interact with its target, AKR1B1, in order to produce the desired therapeutic effects. The drug's mechanism of action may involve modulating the activity of AKR1B1, which is an enzyme involved in various metabolic processes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for epalrestat: AKR1B1 inhibitors
AKR1B1 inhibitors are a type of drugs that target and inhibit the activity of the enzyme AKR1B1. AKR1B1, also known as aldose reductase, is an enzyme involved in the metabolism of glucose and other sugars. It catalyzes the conversion of glucose to sorbitol, which can lead to various pathological conditions when accumulated excessively.
From a biomedical perspective, AKR1B1 inhibitors are of interest because they have the potential to treat or prevent complications associated with diseases such as diabetes and diabetic complications. By inhibiting AKR1B1, these drugs can reduce the conversion of glucose to sorbitol, thereby preventing the accumulation of sorbitol and the subsequent damage to tissues and organs.
AKR1B1 inhibitors can be used as therapeutic agents to target specific diseases or conditions where AKR1B1 activity is implicated. For example, in the context of diabetes, AKR1B1 inhibitors may help prevent or alleviate diabetic neuropathy, retinopathy, and nephropathy, which are complications associated with high glucose levels.
It's important to note that AKR1B1 inhibitors may have potential side effects and interactions with other medications, so their use should be carefully monitored and prescribed by healthcare professionals.
Drug Target R&D Trends for epalrestat
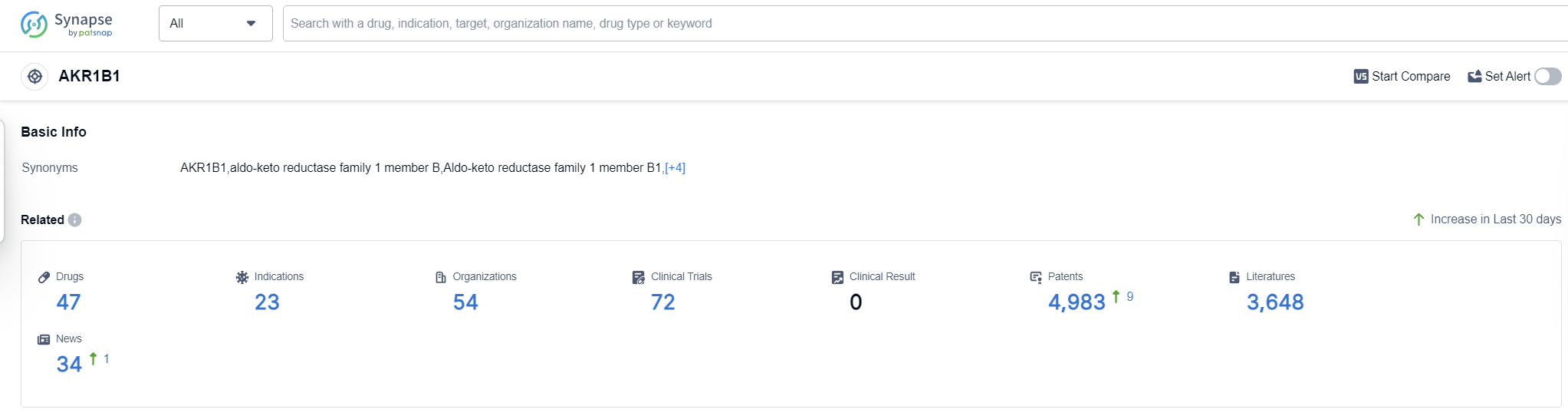
AKR1B1, also known as aldose reductase, is an enzyme that plays a crucial role in the human body. It is primarily involved in the metabolism of glucose and other sugars. AKR1B1 catalyzes the conversion of glucose to sorbitol, which is further metabolized to fructose. This enzyme is found in various tissues, including the liver, kidney, and lens of the eye. While AKR1B1's role in normal glucose metabolism is essential, its overactivity has been linked to various diseases, such as diabetic complications and certain types of cancer. Therefore, understanding and targeting AKR1B1's function can have significant implications in the development of therapeutic interventions in the pharmaceutical industry.
According to Patsnap Synapse, as of 16 Sep 2023, there are a total of 47 AKR1B1 drugs worldwide, from 54 organizations, covering 23 indications, and conducting 72 clinical trials.
Based on the analysis of target AKR1B1, it can be concluded that several companies are showing significant growth and R&D progress in this area. The indications for which drugs have been approved indicate a focus on diabetic neuropathies, diabetes mellitus, cataract, and diabetic cardiomyopathies. Small molecule drugs and monoclonal antibodies are the most rapidly progressing drug types, suggesting potential competition around innovative drugs. China, Japan, the United States, and various countries in the European Union show progress in the development of drugs targeting AKR1B1. Overall, the current competitive landscape indicates a promising future for the development of AKR1B1-targeted drugs.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Epalrestat is a small molecule drug developed by Alfresa Pharma Corp. It targets AKR1B1 and is used in the treatment of nervous system diseases, endocrinology and metabolic diseases, as well as congenital disorders. Its active indications include diabetic neuropathies, diabetes mellitus, and congenital disorder of glycosylation type 1A. Epalrestat has received regulatory approval in Japan and China, and it was first approved in Japan in 1992. As an orphan drug, it is intended to treat rare diseases or conditions.