Equillium Announces Positive Interim Results of Phase 3 Study for Itolizumab in Acute Graft-Versus-Host Disease
Equillium, Inc., a biotechnology firm in the clinical development phase that specializes in creating innovative treatments for serious autoimmune and inflammatory conditions, disclosed that the Independent Data Monitoring Committee has given a favorable recommendation after examining the interim outcomes from the Phase 3 EQUATOR trial assessing itolizumab in patients with acute graft-versus-host disease.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 The Independent Data Monitoring Committee (IDMC) assessed unblinded data from over 100 patients up to Day 29 of treatment in the study. The assessment followed predefined boundaries for futility and efficacy stopping, and the committee advised the continuation of the study without any modifications.
The Independent Data Monitoring Committee (IDMC) assessed unblinded data from over 100 patients up to Day 29 of treatment in the study. The assessment followed predefined boundaries for futility and efficacy stopping, and the committee advised the continuation of the study without any modifications.
“We are satisfied with the interim review results, where the IDMC did not flag any safety or futility issues and recommended the Phase 3 EQUATOR study proceed as planned,” commented Bruce Steel, CEO of Equillium.
“With over 100 clinical sites worldwide actively enrolling patients, we are eager to complete the study promptly. We believe itolizumab could provide significant benefits to patients with acute graft-versus-host disease, a condition with high mortality rates where high-dose corticosteroids remain the standard of care,” added Bruce Steel.
The blinded interim data and the IDMC's recommendation have been shared with our partner, Ono Pharmaceutical. Ono has until the end of October 2024 to exercise its option to acquire our rights to itolizumab, which would entail a one-time payment of JPY 5.0 billion, or roughly $35.0 million based on the exchange rate on August 5, 2024. If Ono opts to exercise its option, Equillium would also be entitled to receive up to USD $101.4 million upon reaching certain clinical, regulatory, and commercialization milestones.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
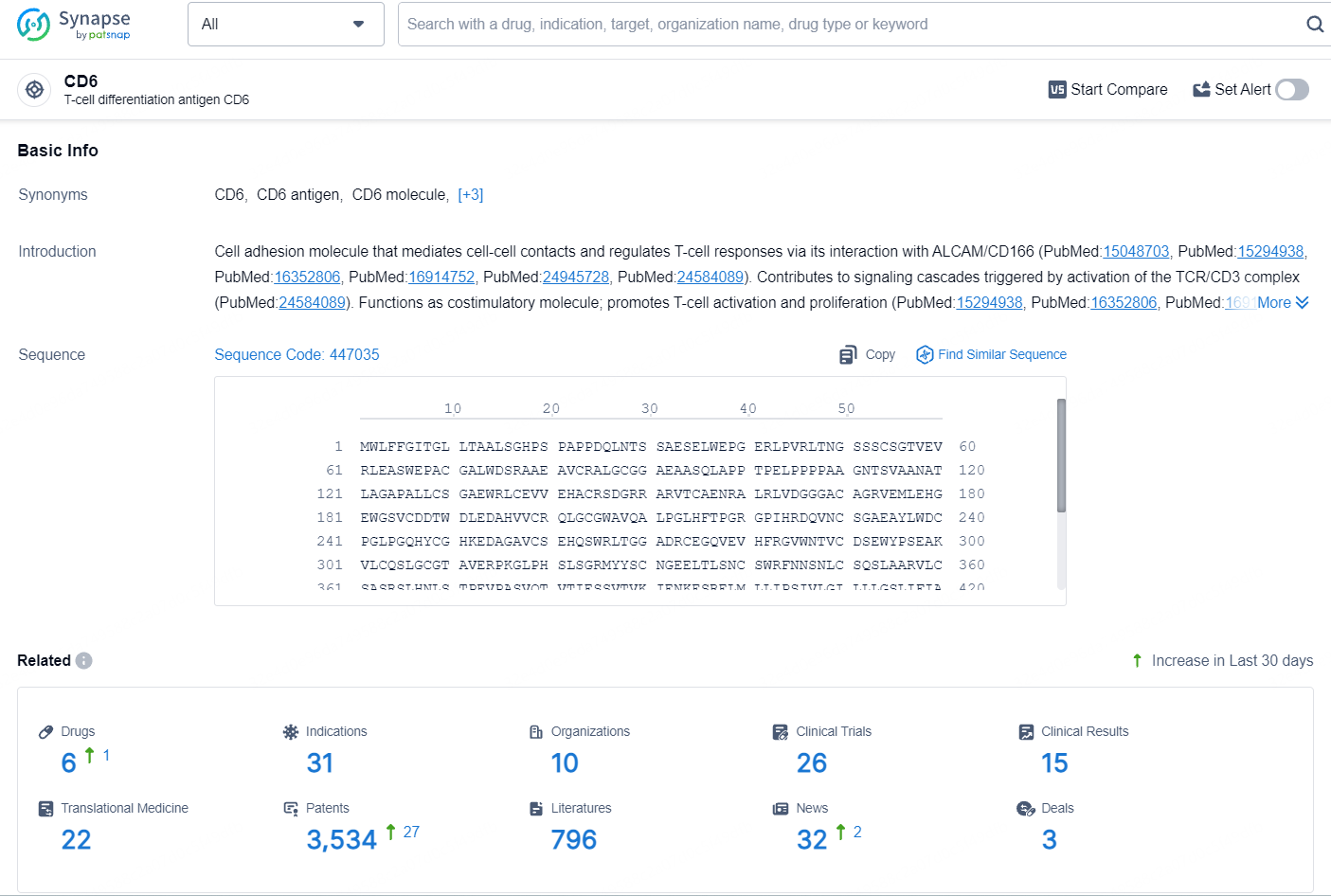
According to the data provided by the Synapse Database, As of August 9, 2024, there are 6 investigational drugs for the CD6 target, including 31 indications, 10 R&D institutions involved, with related clinical trials reaching 26, and as many as 3534 patents.
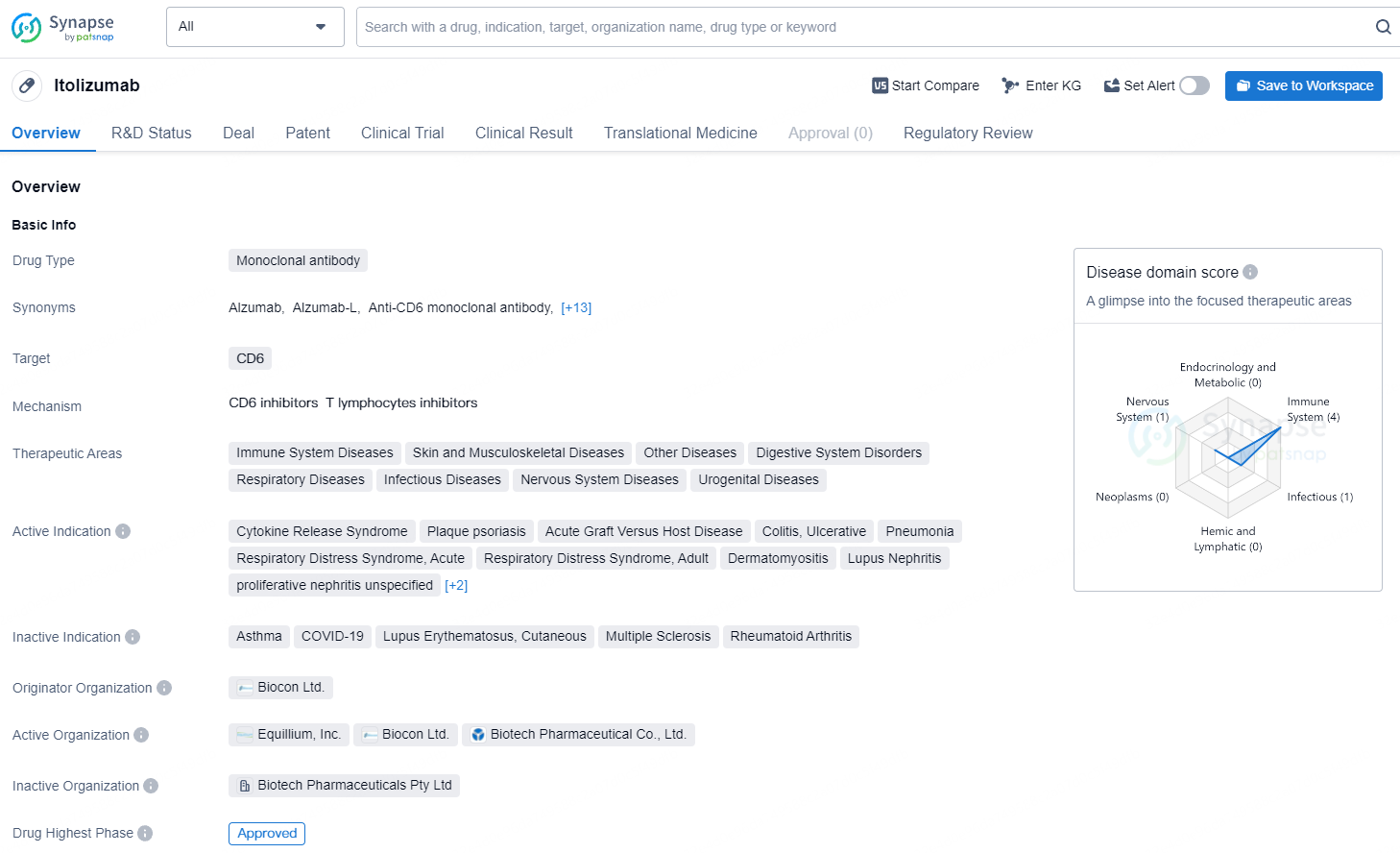
Itolizumab is a clinical-stage, first-in-class anti-CD6 monoclonal antibody that selectively targets the CD6-ALCAM signaling pathway to selectively downregulate pathogenic T effector cells while preserving T regulatory cells critical for maintaining a balanced immune response. This pathway plays a central role in modulating the activity and trafficking of T cells that drive a number of immuno-inflammatory diseases.