EU Approves Atamyo's ATA-200 Human Trials for Limb-Girdle MD 2C/R5
Atamyo Therapeutics recently declared that it has received official approval to commence a clinical study in Europe of its gene therapy product, ATA-200, which is designed for combatting limb-girdle muscular dystrophy Type 2C/R5, a condition associated with the γ-sarcoglycan protein anomaly. This endorsement for the clinical trial protocol was initially given by the regulatory body in Italy, the Italian Medicines Agency, followed by a subsequent nod of approval from the French Medicines Agency.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Our excitement is palpable as we secure authorization from the Clinical Trial Authorization (CTA) in both France and Italy to address the crippling condition known as LGMD2C/R5, which predominantly impacts children and currently lacks any sanctioned treatments," announced Dr. Sophie Olivier, the Chief Medical Officer at Atamyo. "The company is charting plans to commence patient dosing for our investigational gene therapy, ATA-200, in the third quarter of 2024."
"LGMD 2C/R5 represents a grave form of muscular dystrophy that manifests early in life, often resulting in the loss of walking ability before the teenage years," stated Professor Giacomo Comi, the Full Professor of Neurology at the University of Milan, Italy, and the lead researcher for this study. "It is incredibly inspiring to partake in research that could have profound impacts on the lives of those afflicted by this muscular dystrophy."
"A landmark achievement for patients with LGMD-2C/R5 and for our team, the initialization of clinical trials for ATA-200 marks it as the pioneering treatment contender for this specific muscular dystrophy," announced Stéphane Degove, CEO of Atamyo Therapeutics. "Together with the ongoing clinical trials for ATA-100 targeting LGMD2I/R9, this new development underlines Atamyo's dedication and adeptness in introducing revolutionary gene therapies that offer safety and efficacy for individuals with limb-girdle muscular dystrophies."
This pioneering study is an international, initial Phase 1b, non-blinded, dose-ranging investigation to assess the safety, pharmacodynamic properties, effectiveness, and immune response to intravenously administered ATA-200. ATA-200 is a one-time treatment using an Adeno-Associated Virus vector to deliver the human γ-sarcoglycan transgene. During preclinical studies, ATA-200 showed promise with its tolerability and its potential to amend symptoms and clinical markers of the disease. The European Medicines Agency has recognized ATA-200 with the Orphan Medicinal Product Designation.
Concurrently with its gene therapy for LGMD2C/R5, Atamyo is also pioneering a clinical trial for ATA-100 to treat LGMD2I/R9, which stems from a deficit of FKRP, and is actively progressing towards IND (Investigational New Drug) application for addressing LGMD2A/R1, tied to a shortage of the calpain protein.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
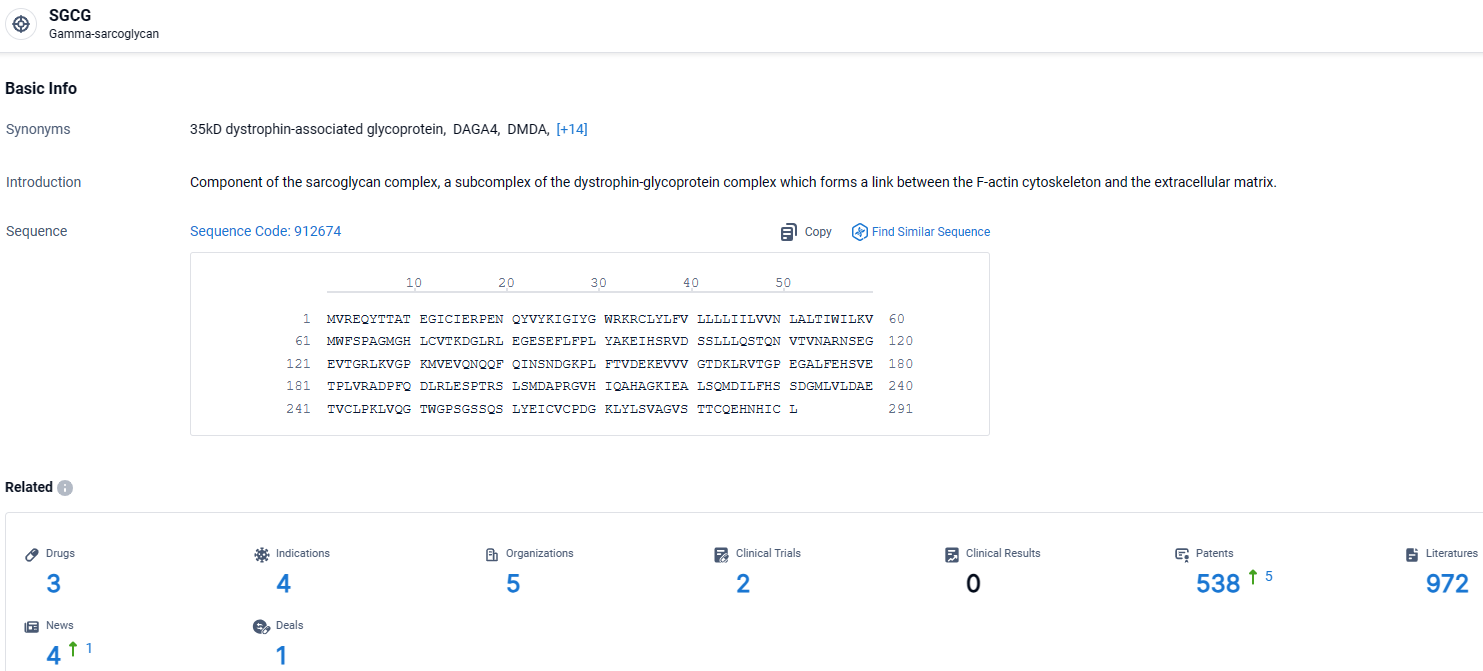
According to the data provided by the Synapse Database, As of March 28, 2024, there are 3 investigational drugs for the SGCG target, including 4 indications, 5 R&D institutions involved, with related clinical trials reaching 2, and as many as 538 patents.
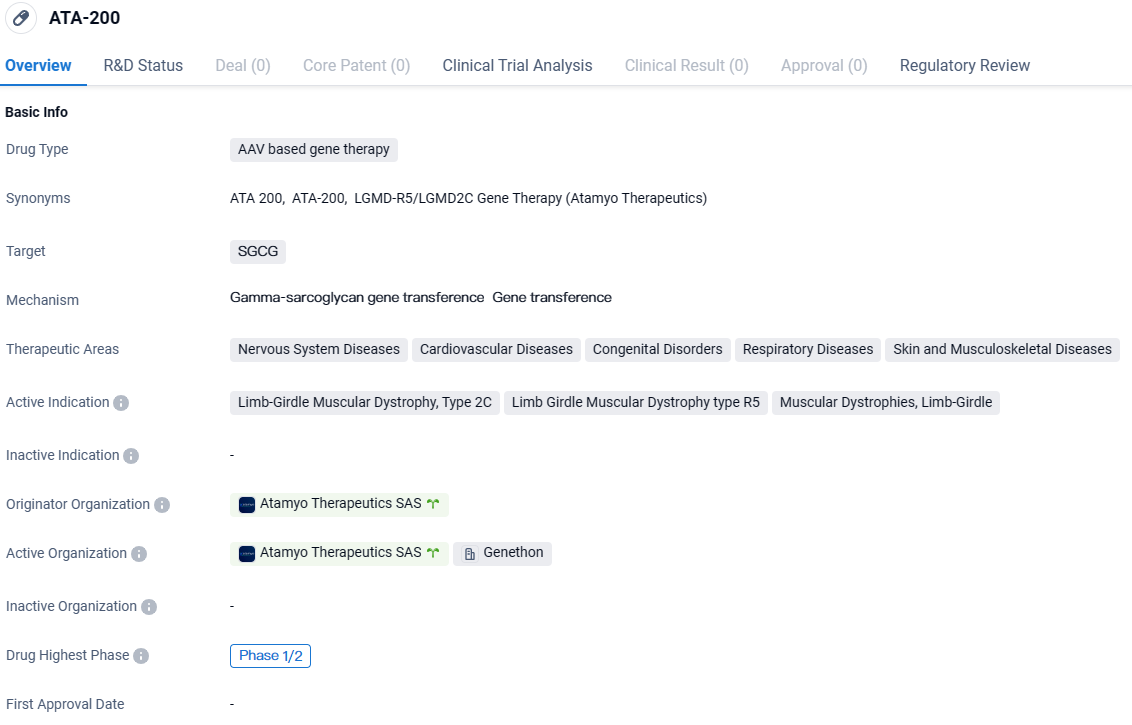
ATA-200 targets SGCG and has shown potential in treating various diseases within the therapeutic areas of Nervous System Diseases, Cardiovascular Diseases, Congenital Disorders, Respiratory Diseases, and Skin and Musculoskeletal Diseases. Specifically, it is being investigated as a treatment for Limb-Girdle Muscular Dystrophy, Type 2C, Limb Girdle Muscular Dystrophy type R5, and Muscular Dystrophies, Limb-Girdle. ATA-200 has been designated as an Orphan Drug and is currently in Phase 1/2 of clinical development.