EU Commission Approves DARZALEX®-SC Quadruplet Regimen for New Multiple Myeloma Patients
Janssen-Cilag International NV, a subsidiary of Johnson & Johnson, has announced that the European Commission (EC) has granted approval for an extension of the indication for DARZALEX® (daratumumab) in its subcutaneous (SC) formulation. This formulation is to be used in combination with bortezomib, lenalidomide, and dexamethasone (referred to as daratumumab-VRd) for patients newly diagnosed with multiple myeloma (NDMM) who qualify for autologous stem cell transplantation (ASCT). This approval allows patients to initiate this quadruplet therapy based on daratumumab SC at the point of initial diagnosis, offering a new treatment option that has been demonstrated to significantly enhance patient outcomes.
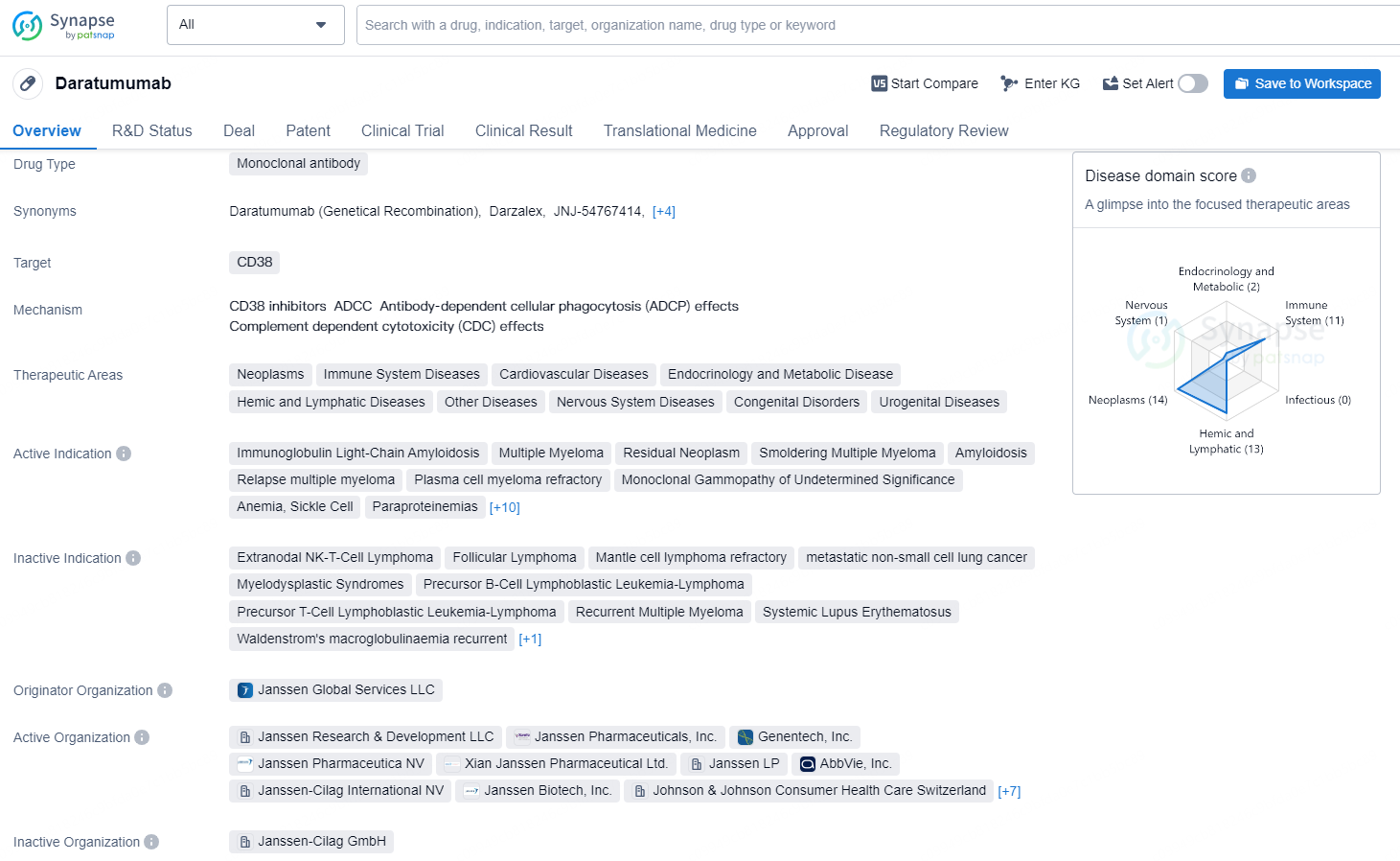
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The approval is based on results from the Phase 3 PERSEUS trial, which analyzed a daratumumab SC-based quadruplet approach for both induction and consolidation therapies, followed by maintenance therapy combining daratumumab SC and lenalidomide (D-R). This was compared to the standard treatment of bortezomib, lenalidomide, and dexamethasone (VRd) used during induction and consolidation phases, subsequently followed by lenalidomide (R) maintenance in a cohort of 709 patients diagnosed with newly diagnosed multiple myeloma (NDMM) eligible for autologous stem cell transplantation (ASCT).
“Multiple myeloma presents as a heterogeneous condition, underscoring the necessity for ongoing advancements in first-line treatment approaches to enhance responses, decrease the likelihood of relapse, and ultimately improve long-term patient outcomes,” remarked Dr. Paula Rodriguez-Otero from the Department of Hematology at the Cancer Center Clínica Universidad de Navarra in Pamplona, Navarra, Spain. “The European Commission's endorsement of the daratumumab SC-based quadruplet regimen introduces a transformative new option, which has demonstrated substantial potential for bettering progression-free survival, increasing complete response rates, and achieving rates of minimal residual disease (MRD) negativity when compared to existing treatment protocols.”
Outcomes from the PERSEUS trial, monitored over a median period of 47.5 months, revealed a significant enhancement in the primary endpoint of progression-free survival (PFS). The daratumumab-VRd combination decreased the risk of disease progression or mortality by 58% in comparison to VRd (hazard ratio [HR], 0.42; 95% confidence interval [CI], 0.30-0.59; p<0.0001). The daratumumab-VRd regimen achieved deeper responses than VRd, displaying an overall MRD-negativity rate at a sensitivity of 10-5 of 75.2% versus 47.5% (p<0.001), a complete response or better rate of 87.9% compared to 70.1% (p<0.001), and sustained MRD negativity for at least 12 months at rates of 64.8% versus 29.7%, respectively.
“The approval of this daratumumab quadruplet regimen by the European Commission signifies a significant advancement in the management of newly diagnosed multiple myeloma,” stated Edmond Chan, MBChB, M.D. (Res), Senior Director and EMEA Therapeutic Area Lead for Hematology at Johnson & Johnson's Innovative Medicine division. “By integrating daratumumab SC into this regimen, we are further enhancing first-line treatments for patients, striving to transform outcomes and establish new care benchmarks for eligible individuals from induction through to maintenance.”
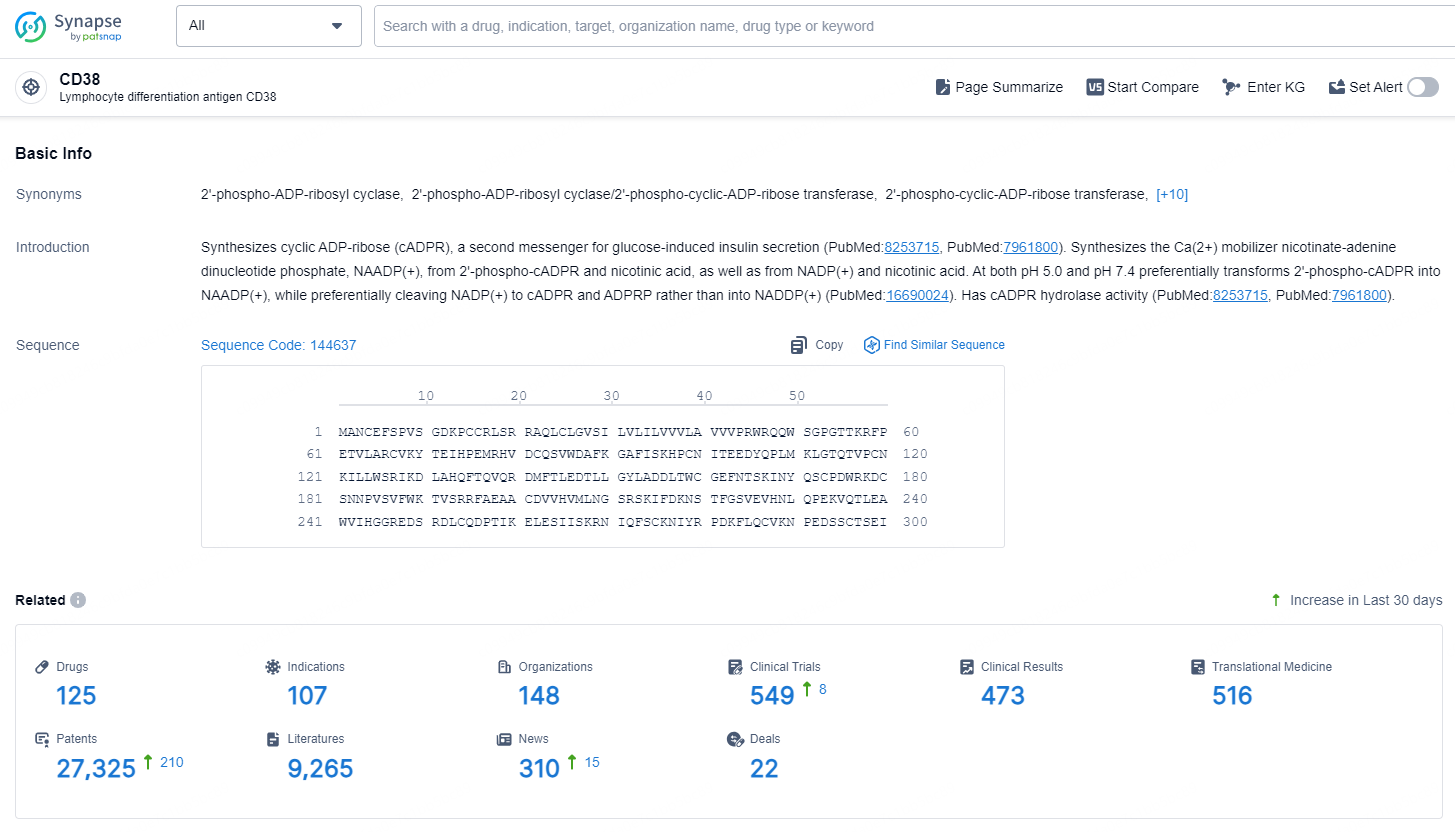
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 125 investigational drugs for the CD38 targets, including 107 indications, 148 R&D institutions involved, with related clinical trials reaching 549, and as many as 27325 patents.
Daratumumab is a monoclonal antibody that targets CD38 and is used in the treatment of various therapeutic areas including neoplasms, immune system diseases, cardiovascular diseases, endocrinology and metabolic disease, hemic and lymphatic diseases, as well as other diseases affecting the nervous system, congenital disorders, and urogenital diseases.