Exploring fosnetupitant's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Fosnetupitant's R&D Progress
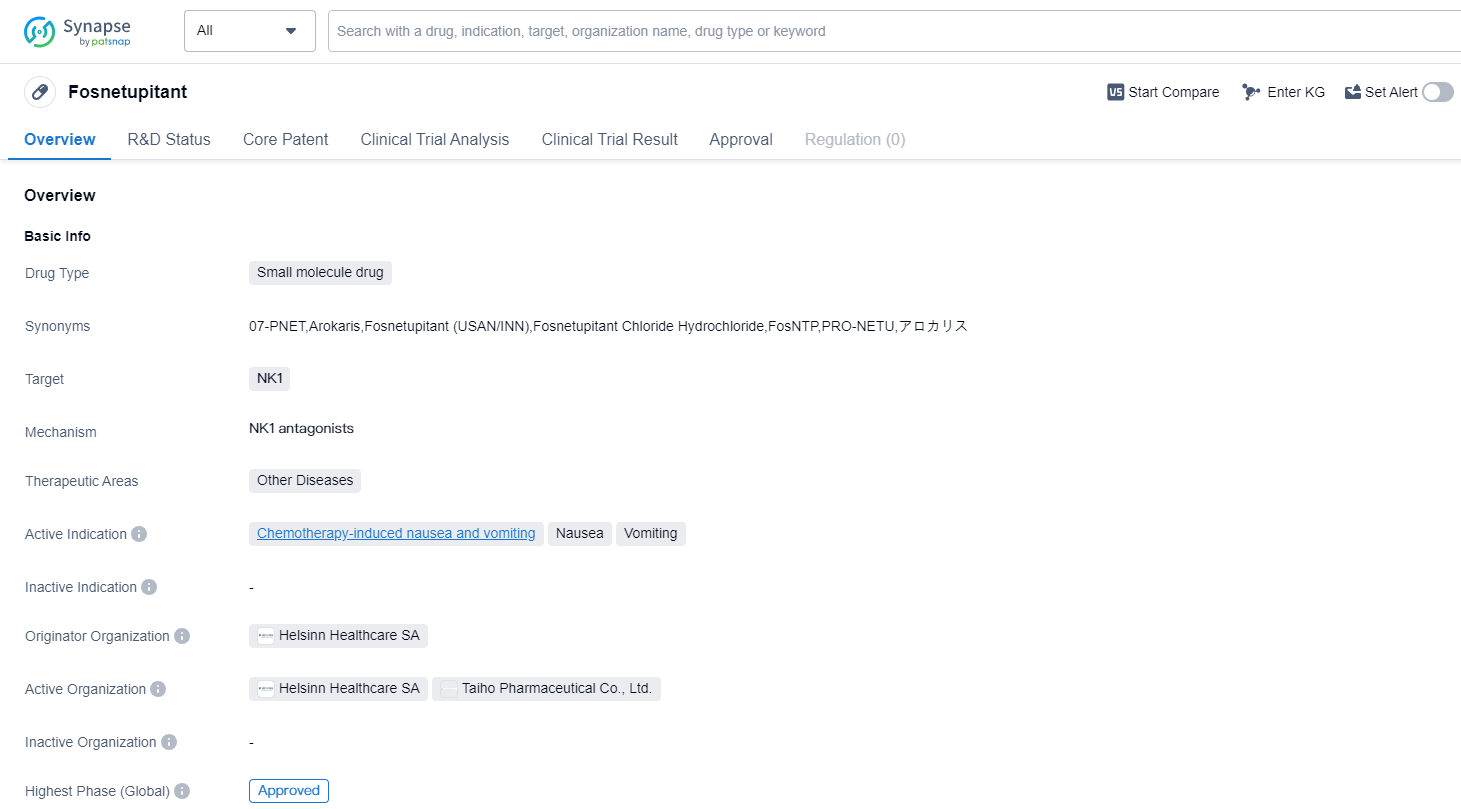
Fosnetupitant is a small molecule drug that falls under the therapeutic area of Other Diseases in the field of biomedicine. It specifically targets the NK1 receptors in the body. The drug has been primarily developed to address chemotherapy-induced nausea and vomiting.
The originator organization behind Fosnetupitant is Helsinn Healthcare SA. This organization has successfully taken the drug through the necessary clinical trials and regulatory processes, resulting in its highest phase of development being approved. This indicates that Fosnetupitant has met the required safety and efficacy standards to be considered a viable treatment option.
The drug received its first approval in Japan in March 2022. This signifies that the Japanese regulatory authorities have reviewed the clinical data and granted permission for Fosnetupitant to be marketed and used within the country. It is important to note that this approval is specific to Japan and does not necessarily imply approval in other countries or regions.
Fosnetupitant's approval for chemotherapy-induced nausea and vomiting is particularly significant, as this is a common and distressing side effect experienced by cancer patients undergoing chemotherapy. By targeting the NK1 receptors, Fosnetupitant aims to alleviate these symptoms and improve the overall quality of life for patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for fosnetupitant: NK1 antagonists
NK1 antagonists are a type of drug that act as antagonists to the neurokinin-1 (NK1) receptor. The NK1 receptor is a protein found in the central nervous system and is involved in transmitting signals related to pain, inflammation, and stress. By blocking the NK1 receptor, NK1 antagonists can inhibit the binding of substance P, a neurotransmitter that plays a role in these processes.
From a biomedical perspective, NK1 antagonists are primarily used in the field of neuropharmacology. They have shown efficacy in the treatment of various conditions such as chemotherapy-induced nausea and vomiting, postoperative nausea and vomiting, and psychiatric disorders like anxiety and depression. By blocking the NK1 receptor, these antagonists can help alleviate symptoms associated with these conditions.
It's important to note that NK1 antagonists should be used under medical supervision, as they may have potential side effects and interactions with other medications. The specific mechanism of action and dosage of each NK1 antagonist may vary, so it is crucial to consult a healthcare professional for appropriate use.
Drug Target R&D Trends for fosnetupitant
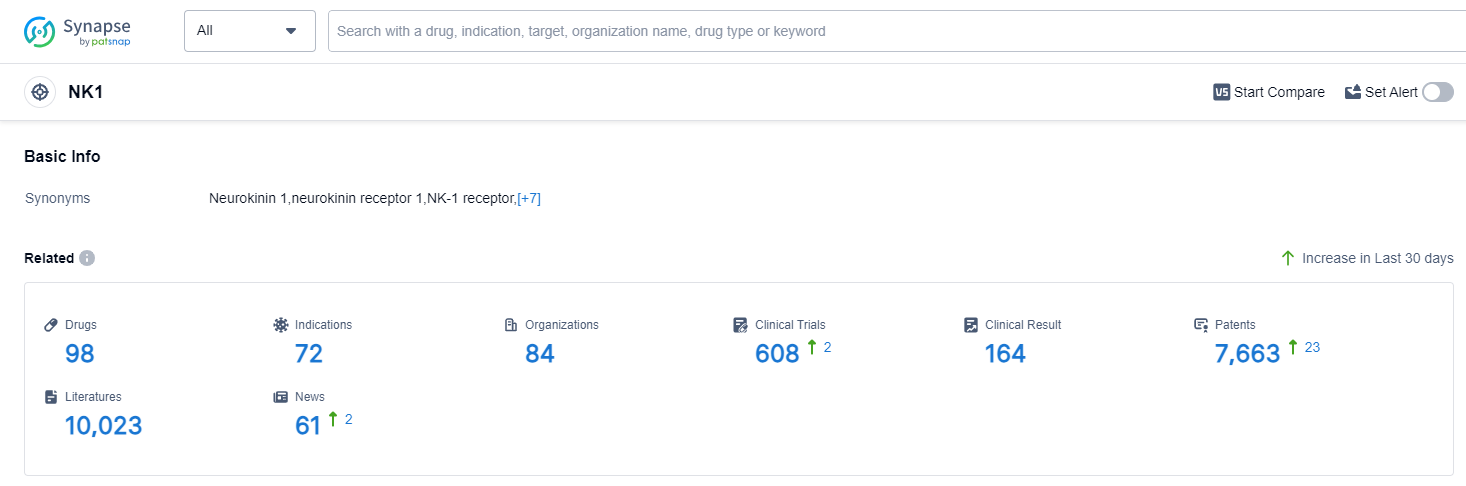
NK1, also known as neurokinin-1 receptor, plays a crucial role in the human body. It is a G-protein coupled receptor that is primarily found in the central nervous system and peripheral tissues. NK1 is involved in various physiological processes, including pain transmission, inflammation, mood regulation, and vomiting. Activation of NK1 by substance P, its primary ligand, leads to the release of neurotransmitters and neuropeptides, resulting in the modulation of these processes. Due to its involvement in multiple pathways, targeting NK1 has become an attractive approach for the development of pharmaceutical interventions in conditions such as pain, depression, and chemotherapy-induced nausea and vomiting.
According to PatSnap Synapse, as of 14 Sep 2023, there are a total of 98 NK1 drugs worldwide, from 84 organizations, covering 72 indications, and conducting 608 clinical trials.
Based on the analysis of the data, the current competitive landscape of target NK1 in the pharmaceutical industry is characterized by the presence of multiple companies with varying stages of development and drug types. Merck & Co., Inc. is leading in terms of the highest stage of development, with several approved drugs. The indications with the highest number of approved drugs are chemotherapy-induced nausea and vomiting, nausea, and vomiting. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, European Union, and Japan are the leading countries/locations in the development of drugs under target NK1, with China also showing progress. Overall, the future development of target NK1 in the pharmaceutical industry is promising, with potential for further advancements in drug development and approvals.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, as a small molecule drug targeting NK1 receptors, it has been approved for use in Japan to address chemotherapy-induced nausea and vomiting, as well as general nausea and vomiting. The drug's approval marks an important milestone in its development and offers hope for patients experiencing these debilitating symptoms.