Exploring Osimertinib Mesylate: Advances and Innovations in Targeted Non-Small Cell Lung Cancer Therapy
Osimertinib mesylate is a small molecule drug that targets EGFR L858R, EGFR T790M, and EGFR-Ex19del. It is indicated for the treatment of a wide range of neoplasms, respiratory diseases, nervous system diseases, digestive system disorders, and other diseases. Specifically, it is approved for the treatment of non-small cell lung cancer with various mutations, including EGFR exon 19 deletions, EGFR exon 21 substitution, EGFR T790M mutation, and EGFR ex20ins mutation. It is also approved for the treatment of metastatic non-small cell lung cancer, unresectable lung non-small cell carcinoma, and various stages of non-small cell lung cancer.
The drug was first approved by the United States in November 2015 and is developed by AstraZeneca Pharmaceuticals Co. Ltd. It has received various regulatory designations, including priority review, accelerated approval, fast track, accelerated assessment, breakthrough therapy, orphan drug, and special review project.
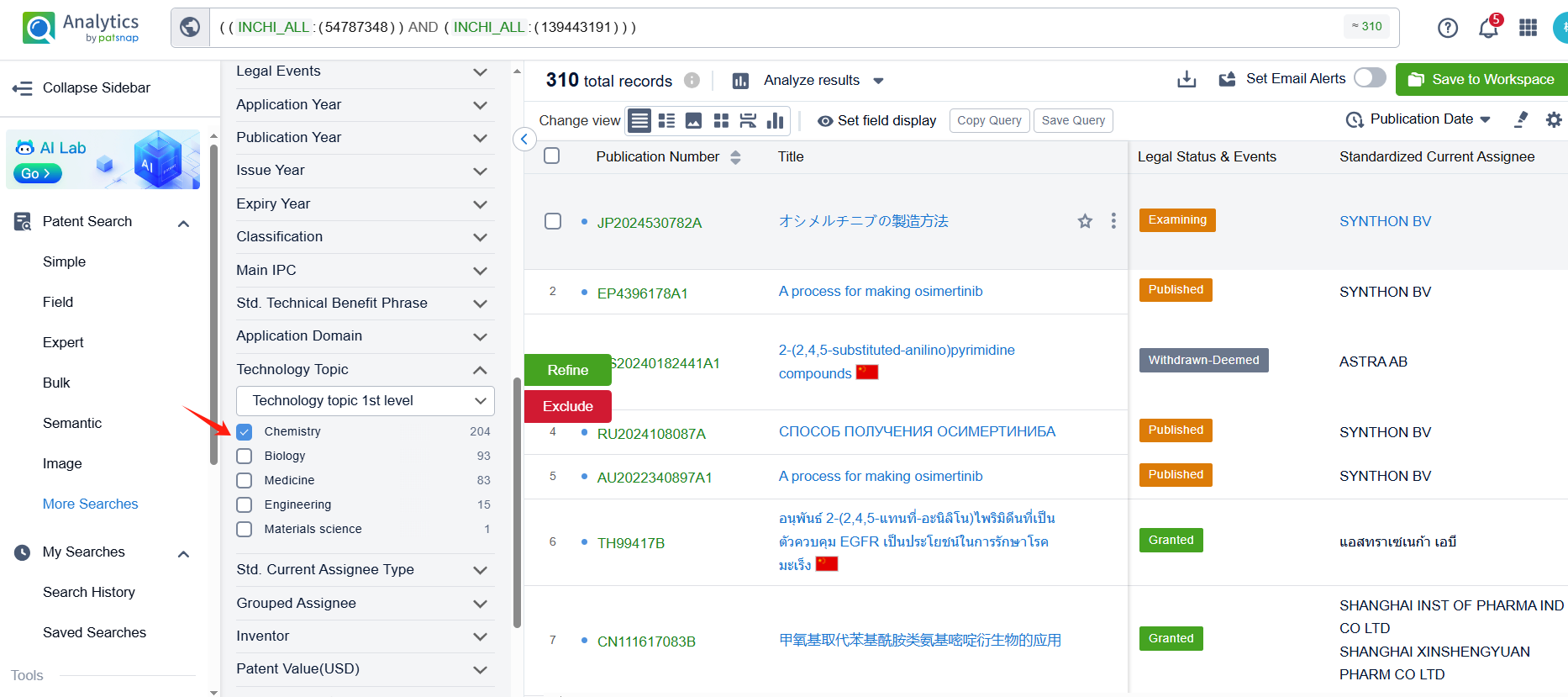
Log in to the Patsnap Chemical. Select the Multi-Structure search, and search for the reactants and products in the specific steps of the synthesis route of Osimertinib. click on search references, and you can query the relevant literature.
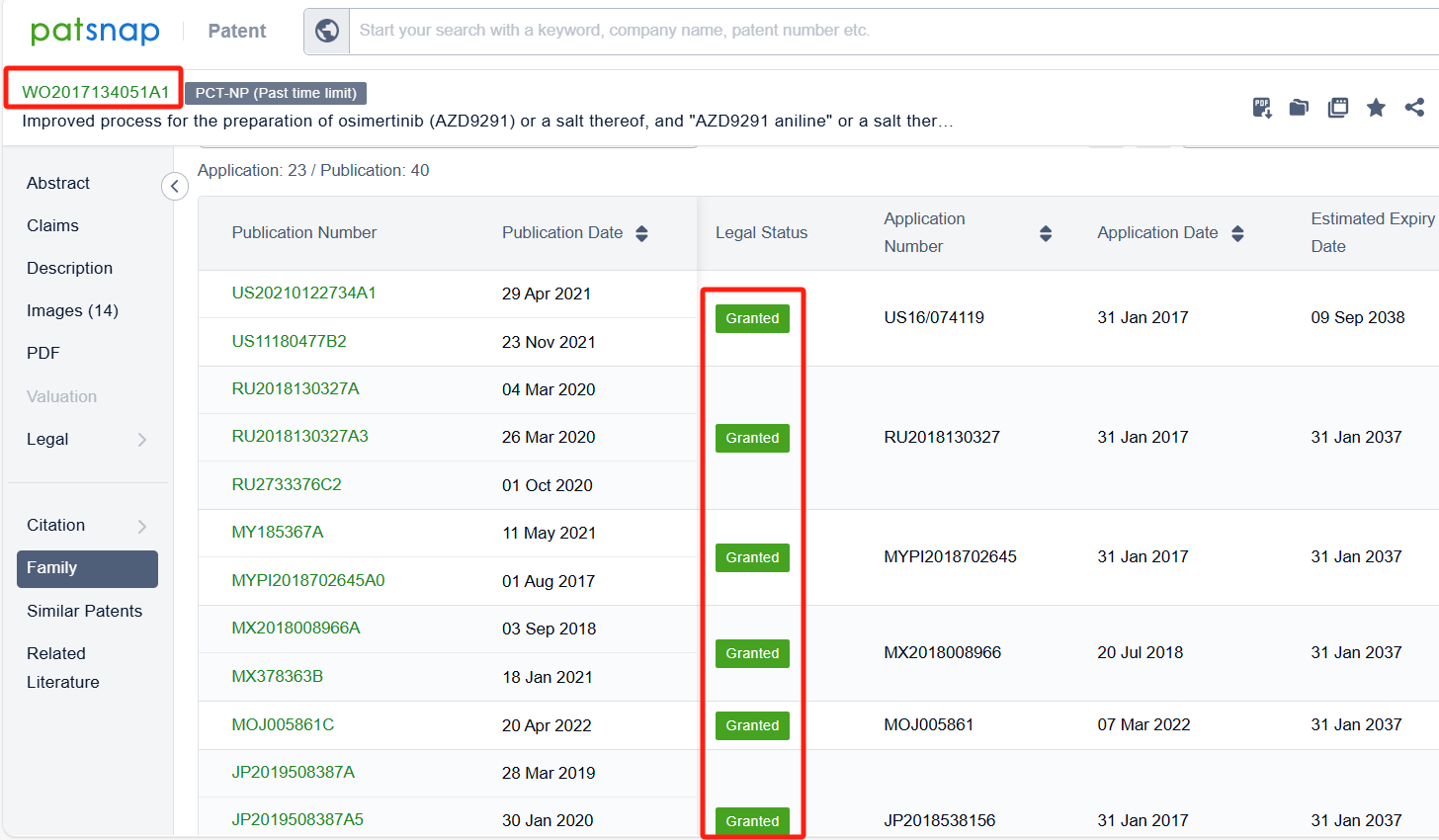
Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Osimertinib process route by AstraZeneca PLC and other companies,Such as AstraZeneca PLC's international patent WO2017134051A1 (application date 20170131, publication date 20170810) describes an improved process for the preparation of a compound called AZD9291 Aniline, which is a key intermediate in the development of the drug osimertinib. The new process avoids the formation of a troublesome impurity called AZD9291 Aniline Hydroxy, which can lead to further degradation products during the synthesis of AZD9291 Aniline. Its corresponding patents in China, Japan, South Korea, and the United States have all been granted.
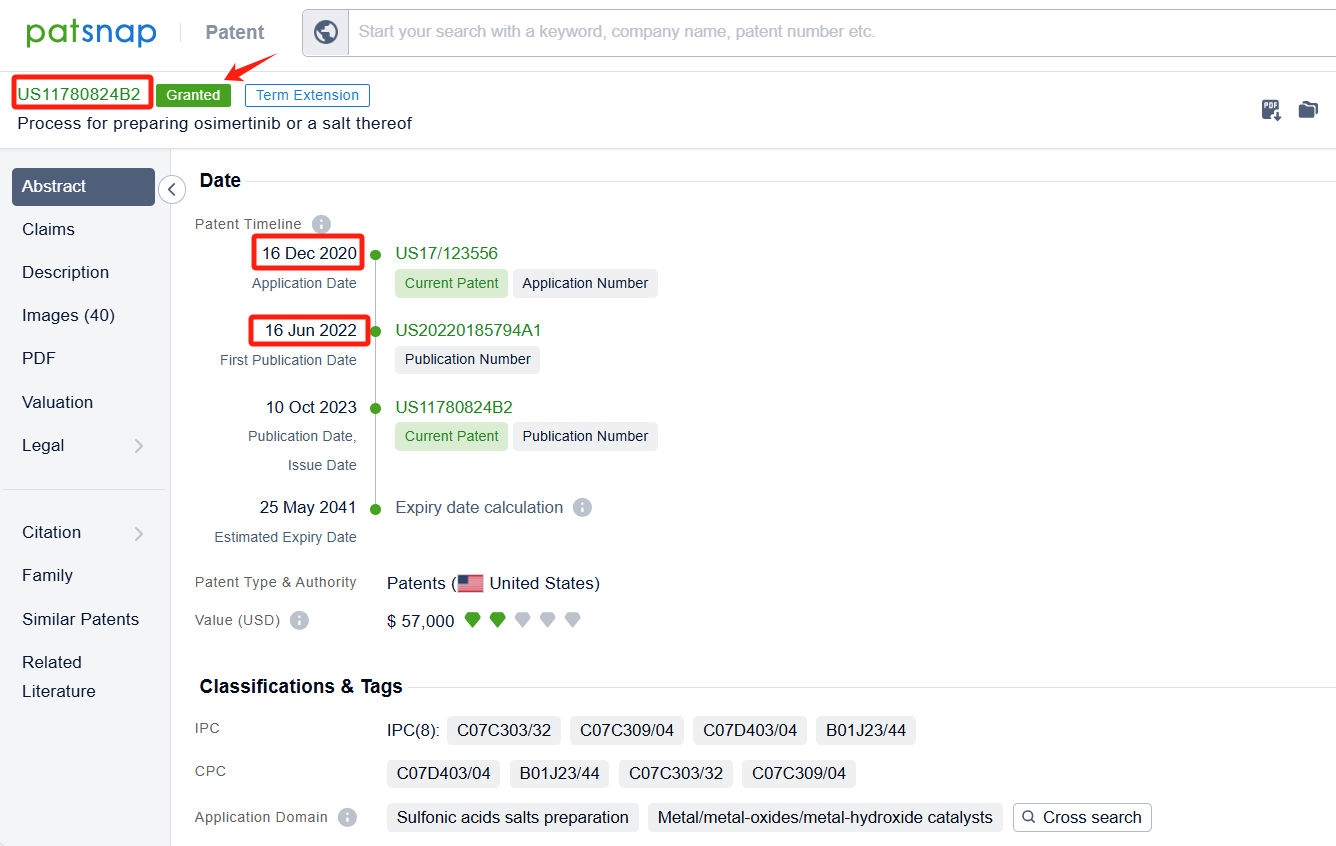
ScinoPharm Taiwan Ltd.'s patent US11780824B2 (application date 20201216, publication date 20220616) provides three methods for making the drug osimertinib and its salt. The first method involves a one-step process where a hydrogen source and a transition-metal catalyst are used to form a compound, which is then reacted with another compound to form osimertinib. The second method involves a two-step process where a different compound is first formed and then converted to osimertinib. The third method involves a three-step process where a different compound is first formed and then converted to another compound before being converted to osimertinib. The patent was granted on October 10, 2023, with an expiration date of May 25, 2041. Additionally, Synthon BV's patent AU2022340897A1 (application date 20220901, publication date 20240314) describes a process for preparing a specific compound (Osimertinib) or a salt thereof. This process involves several steps, including reacting one compound with another in the presence of a catalyst and a solvent.
Osimertinib mesylate shows promise in the treatment of a wide range of cancers, particularly non-small cell lung cancer with specific genetic mutations. Its approval in multiple countries and regulatory designations reflect the significance of the drug in treating these conditions. As an expert in the pharmaceutical industry, it is crucial to continue monitoring the drug's performance in the market and its potential for further indications or developments in the field of biomedicine.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.