Exploring Riluzole's R&D successes and its clinical results at the 2024 ASCO_GI
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the US. 5-fluorouracil (5-FU) based chemotherapies in combination with targeted agents remain the standard of care in patients with metastatic or locally advanced disease. It is essential to develop new treatment strategies in metastatic CRC patients with microsatellite stable disease. And the promising clinical anti-tumor effect of Riluzole on CRC were presented in 2024 ASCO_GI.
Riluzole's R&D Progress
Riluzole is a small molecule drug that primarily targets SCNA. It is used in the treatment of various therapeutic areas including Nervous System Diseases, Endocrinology and Metabolic Disease, Neoplasms, Digestive System Disorders, and Skin and Musculoskeletal Diseases. The drug has been approved for the treatment of Amyotrophic Lateral Sclerosis, Metastatic Colorectal Carcinoma, Advanced Malignant Solid Neoplasm, Melanoma recurrent, Melanoma, Cutaneous Malignant, and Refractory Malignant Solid Neoplasm.
According to the Patsnap Synapse, Riluzole has reached the highest phase of development, which is approved globally. And the clinical trial distributions for Riluzole are primarily in the United States, China and United Kingdom. The key indication is Amyotrophic Lateral Sclerosis. 
Detailed Clinical Result of Riluzole
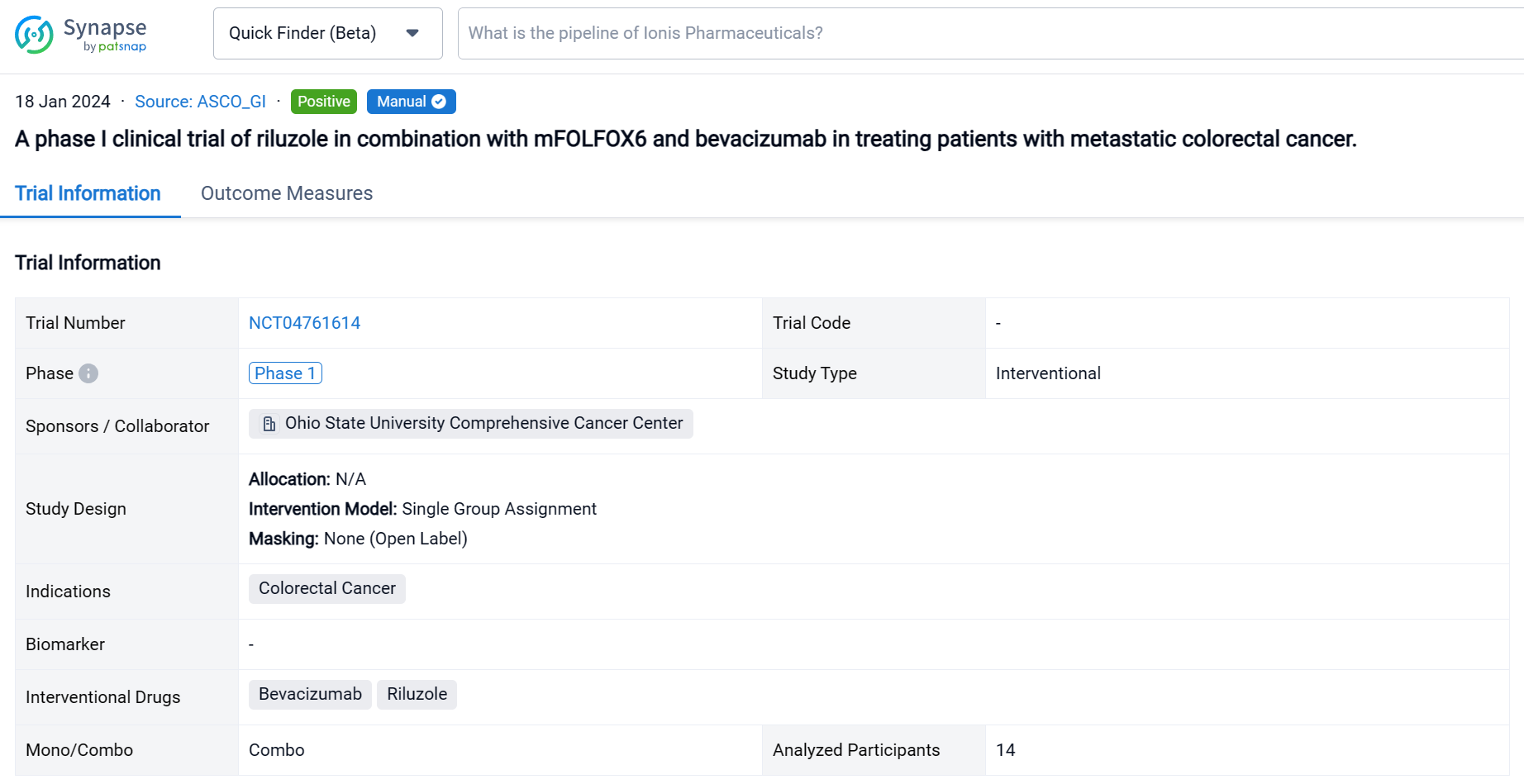
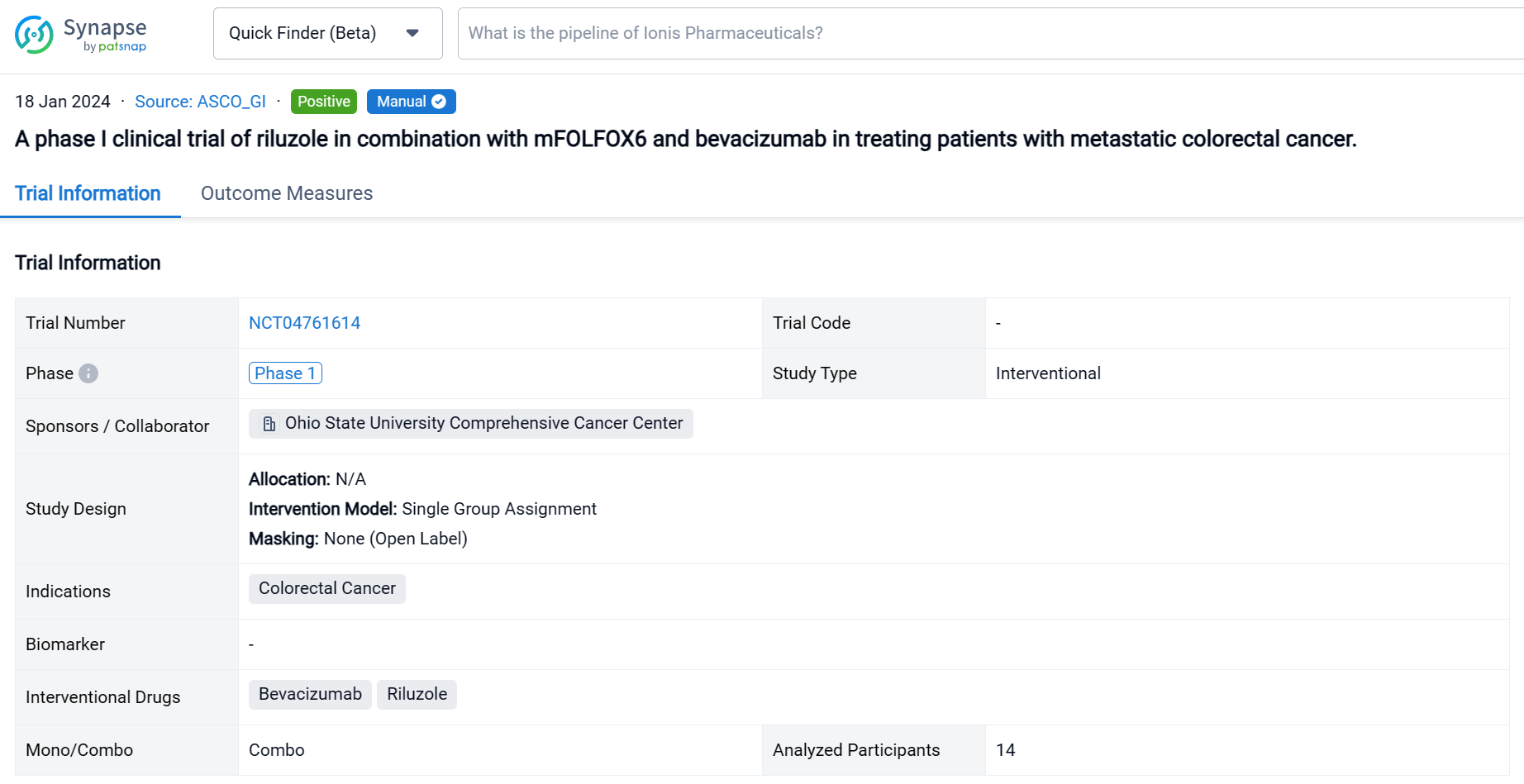
This single group assignment, open-labeled clinical trial (NCT04761614) was aimed to assess the efficacy and safety of riluzole in combination with mFOLFOX6/bevacizumab for patients with metastatic CRC.
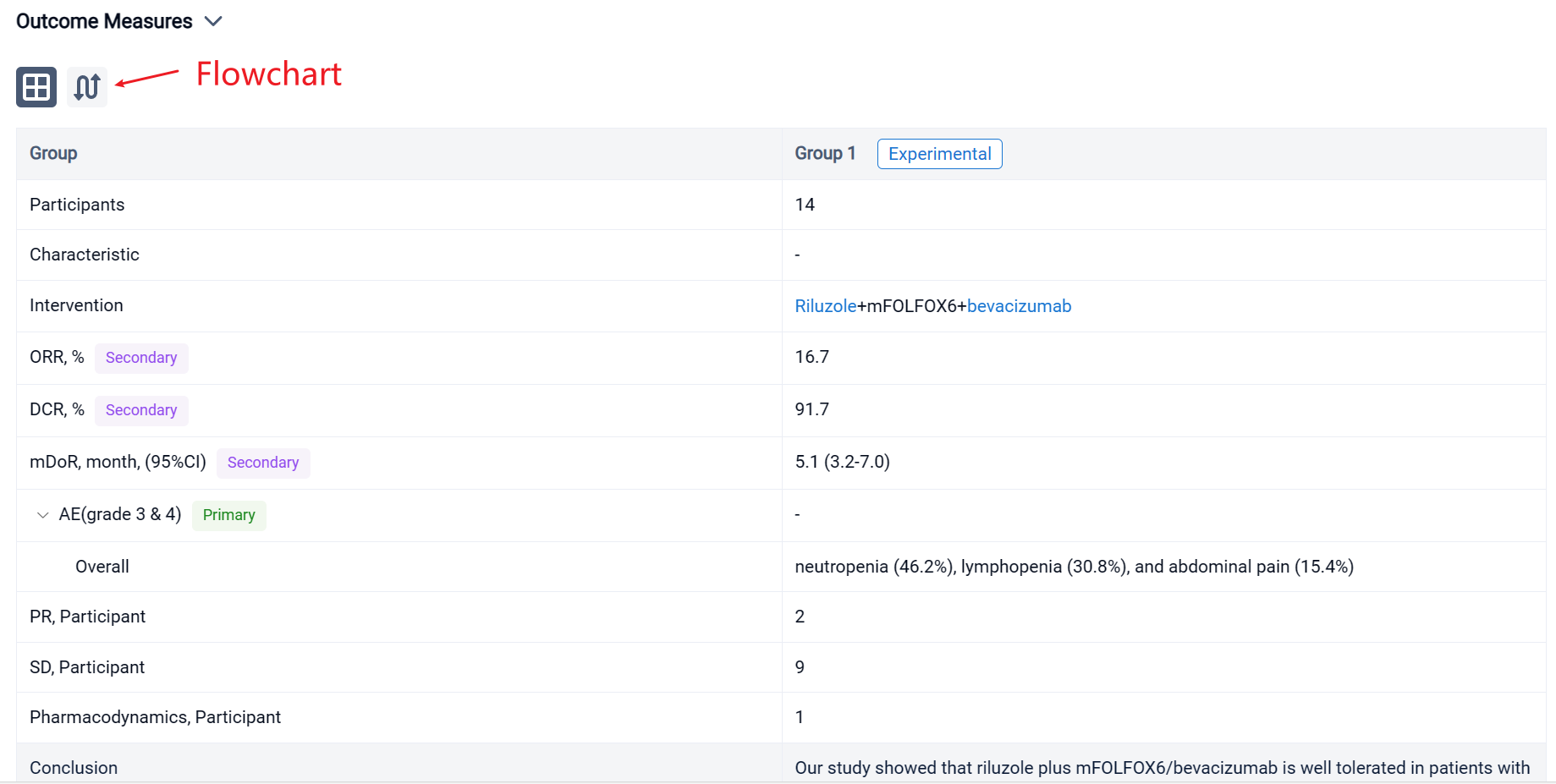
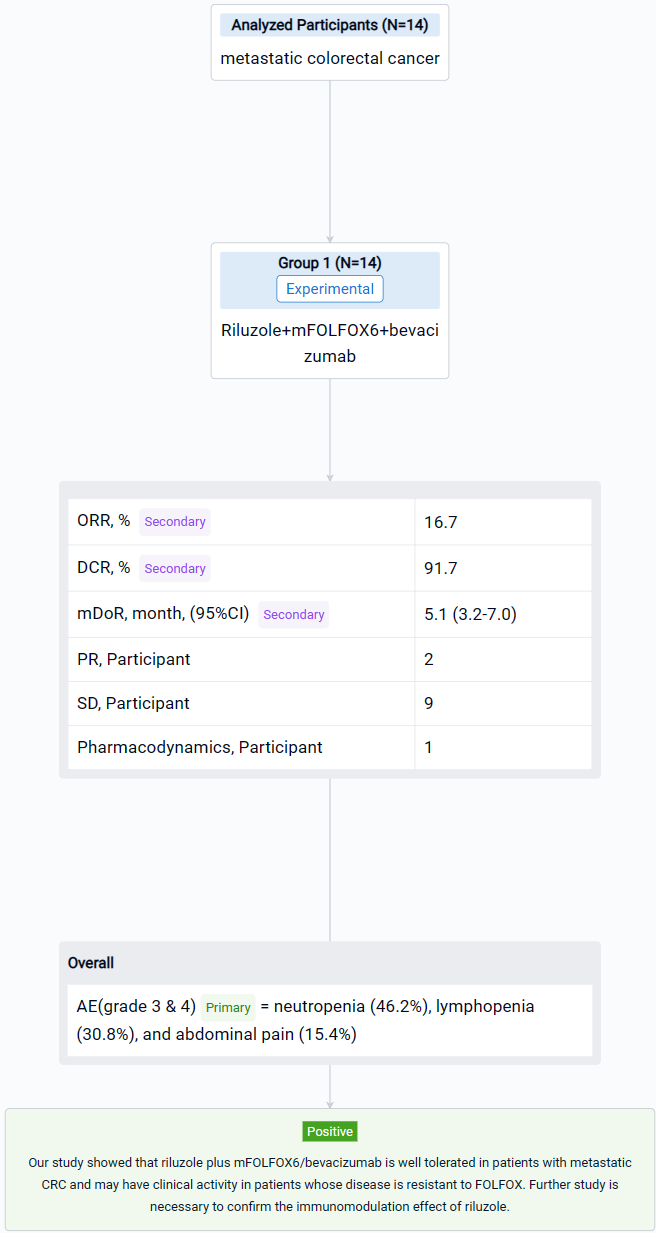
In this study, the dose of riluzole started from 50 mg twice daily with dose escalation to 100 mg twice daily or dose de-escalation to 50 mg once daily. Patients received riluzole for 16 weeks in combination with mFOLOFX6/bevacizumab for 8 cycles. Then patients either continued mFOLFOX/bevacizumab or switched therapy. The primary objective was adverse events (AE). The secondary objectives were objective response rate (ORR), disease control rate (DCR), and duration of response (DoR).
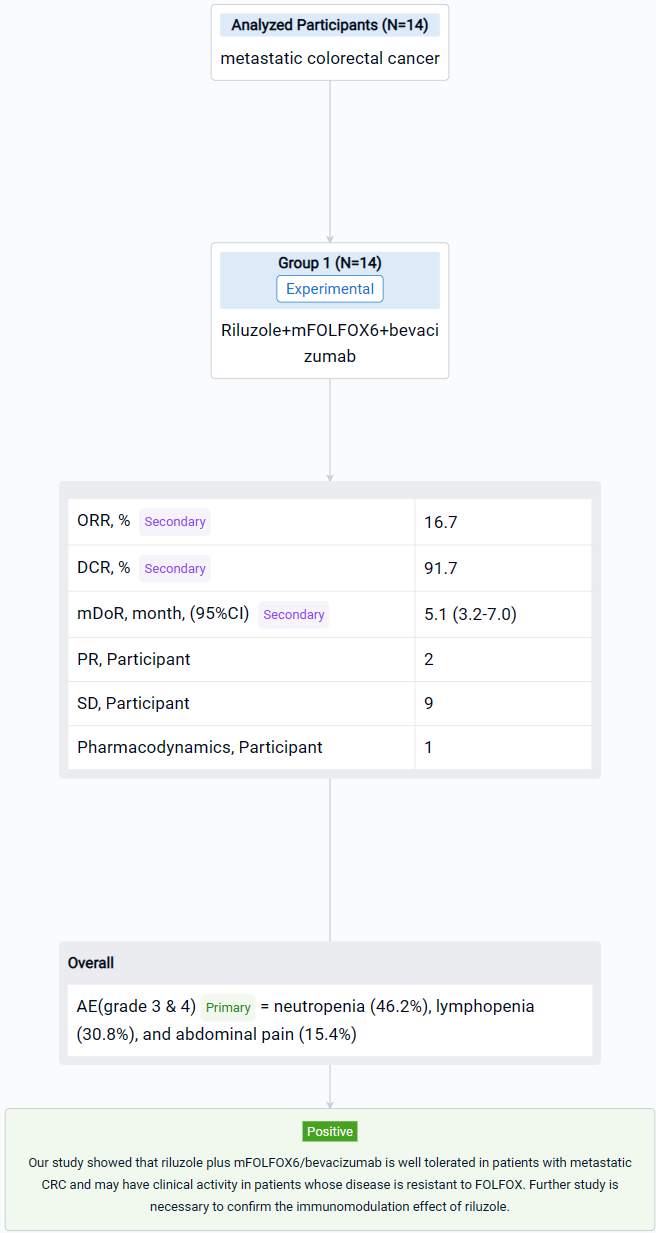
The result showed that fourteen patients were enrolled, and twelve patients were evaluable. All patients received FOLFOX in the past; two patients received 2 lines of chemotherapies; one patient received 3 lines and eight patients had ≥ 4 lines before the study. Five patients (41.7%) had disease resistance to FOLFOX. During the study, two patients received only 2 cycles of treatment due to poor PS. Two patients received 7 cycles, and nine patients completed 8 cycles of treatment. The common grade 3 & 4 AEs included neutropenia (46.2%), lymphopenia (30.8%), and abdominal pain (15.4%). No complete response was observed; two patients obtained partial response; nine patients had stable disease, and only one patient had progressive disease. The ORR was 16.7%, and DCR was 91.7%. The median DoR was 5.1 (95% CI 3.2-7.0) months. The maximum tolerated dose for riluzole is 100 mg twice daily.
It can be concluded that riluzole plus mFOLFOX6/bevacizumab is well tolerated in patients with metastatic CRC and may have clinical activity in patients whose disease is resistant to FOLFOX. Further study is necessary to confirm the immunomodulation effect of riluzole.
How to Easily View the Clinical Results Using Synapse Database?
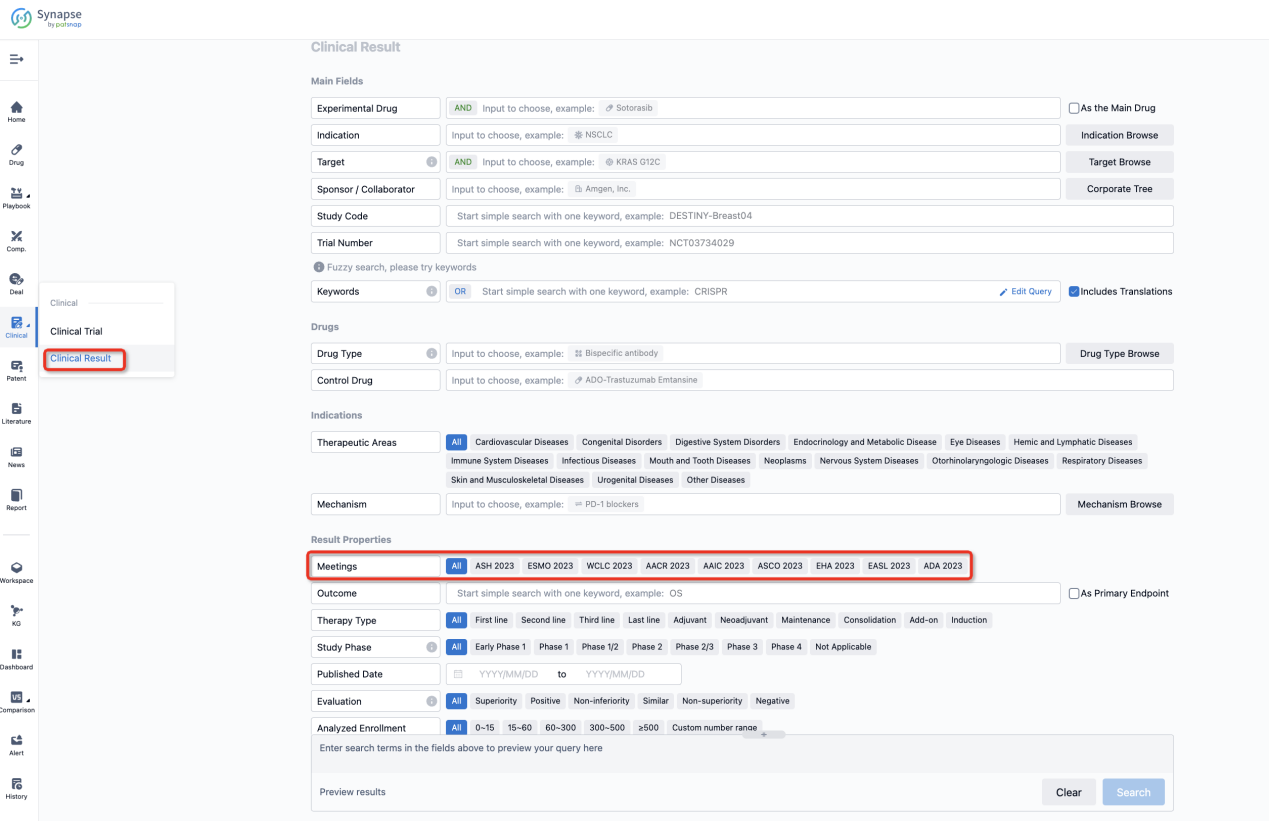
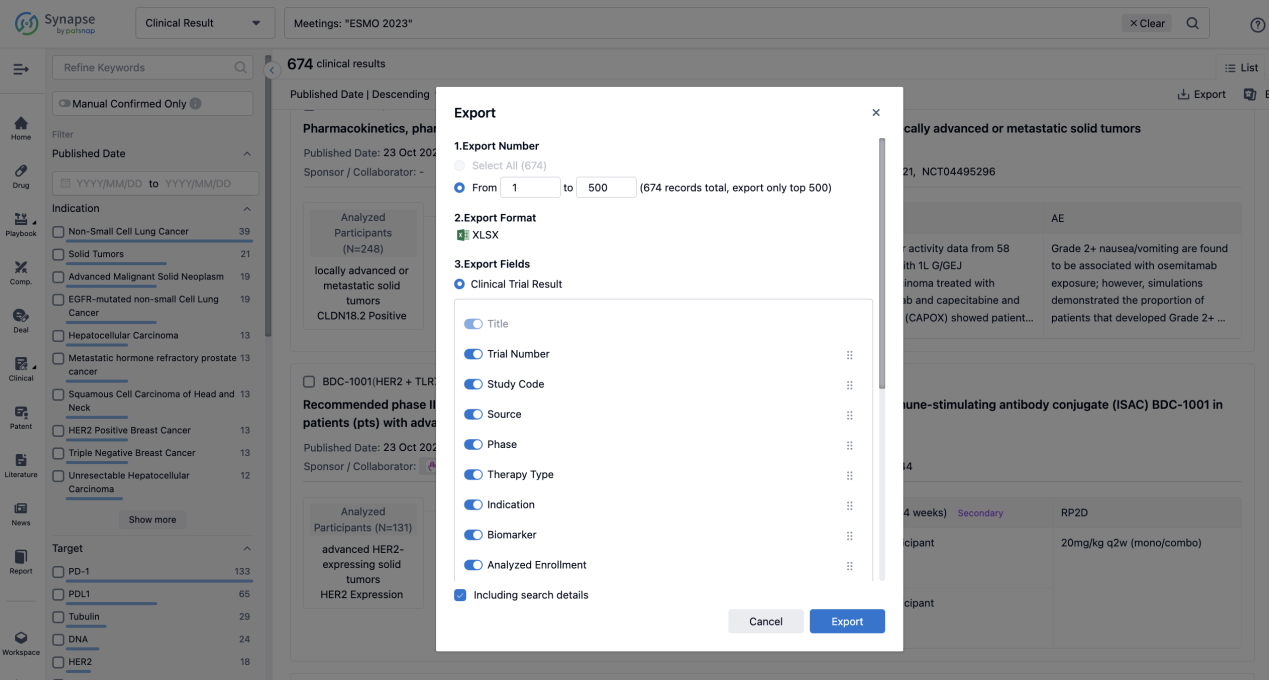
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
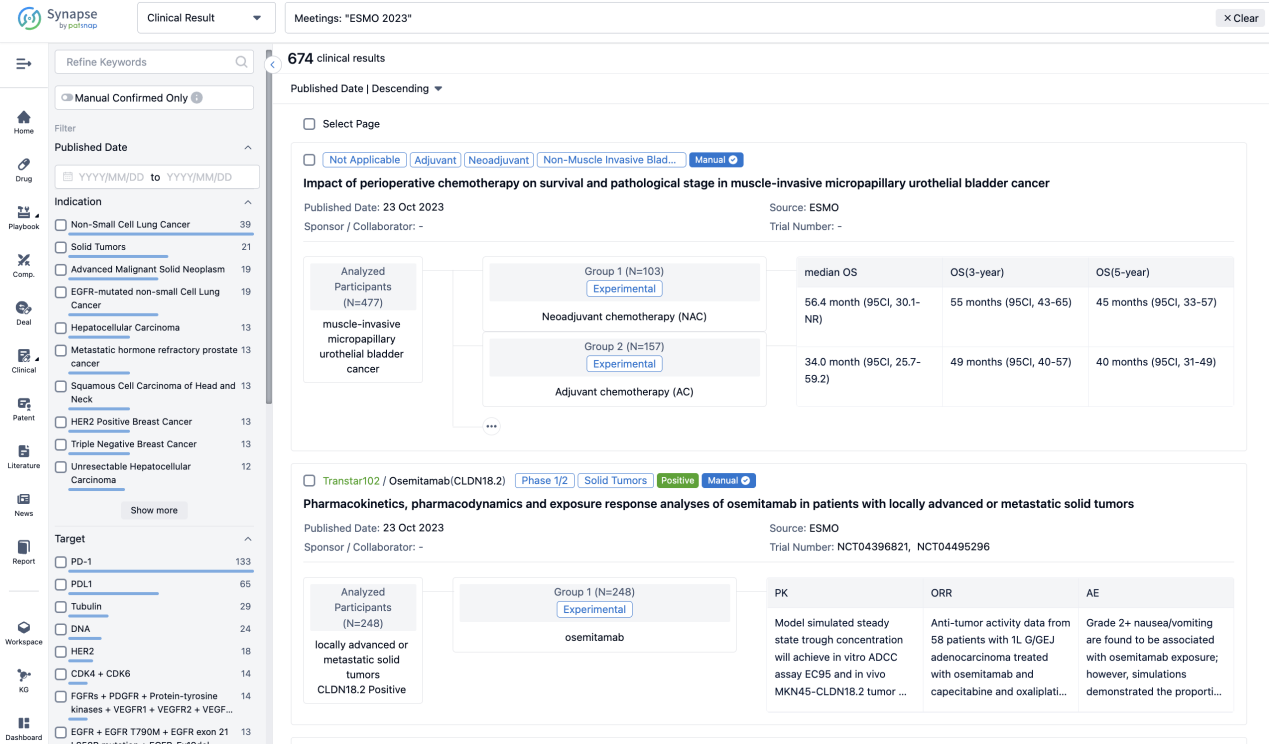
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!