Exploring the Latest AGT siRNA Deal by Sanegene Bio (Suzhou): A Guide to Rapidly Accessing Transaction Insights
On December 27, 2023, Innovent Biologics, Inc. and Sanegene Bio (Suzhou) Co., Ltd. jointly announced that they have entered into a strategic collaboration agreement to co-develop SGB-3908, a small interfering RNA (siRNA) candidate drug targeting angiotensinogen (AGT) for the treatment of hypertension. According to the terms of the agreement, Innovent has obtained the exclusive option to acquire rights for the exclusive development, production, and commercialization of SGB-3908 in various global regions upon payment of an option exercise fee in the future. Following the exercise of this option by Innovent, Sanegene Bio will also be entitled to subsequent research and development milestone payments, sales milestone payments, and tiered royalties based on net sales after commercialization. The companies have not disclosed the financial terms of the collaboration.
About SGB-3908
The drug SGB-3908 is a small interfering RNA (siRNA) that targets AGT, a gene associated with cardiovascular diseases. Specifically, it is being developed for the treatment of hypertension, a common cardiovascular condition characterized by high blood pressure. The drug is currently in the preclinical phase, which means it is still undergoing laboratory testing and has not yet progressed to human clinical trials. Click the image below to directly embark on the exploration journey with the SGB-3908!
About Sanegene Bio (Suzhou) Co., Ltd.
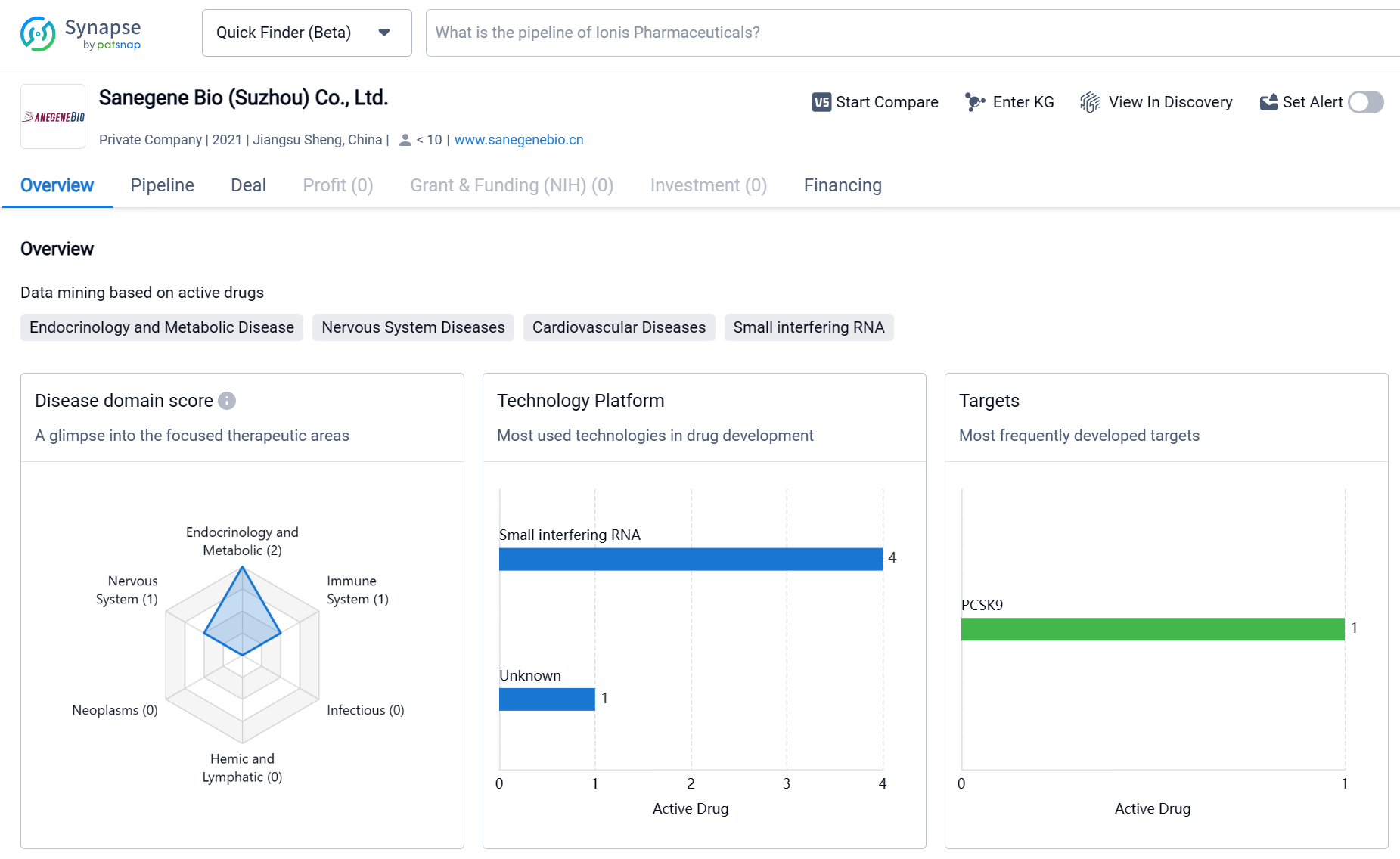
Sanegene Bio (Suzhou) Co., Ltd. is a biomedicine organization based in Jiangsu Sheng, China. The company has focused on developing drugs in various therapeutic areas, with a particular emphasis on Endocrinology and Metabolic Disease. The most frequently developed target by the company is PCSK9, indicating its interest in cardiovascular diseases. The pipeline of Sanegene Bio (Suzhou) Co., Ltd. shows ongoing research and development efforts, with several drugs in the discovery phase. However, there are no drugs in advanced stages of development or regulatory approval as of the given date.
By leveraging the LEAD™ (Ligand and Enhancer Assisted Delivery) platform, Sanegene Bio's innovative design of three modules, namely, tissue-specific delivery ligands, delivery enhancers, and optimal chemical modifications, has enabled Sanegene Bio's powerful engine for RNAi drug discovery to overcome multiple barriers to RNAi drug discovery; and enable the efficient design, screening, characterization, and development of new, efficacious, and safe RNAi molecules with enhanced drug distribution in tissues.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
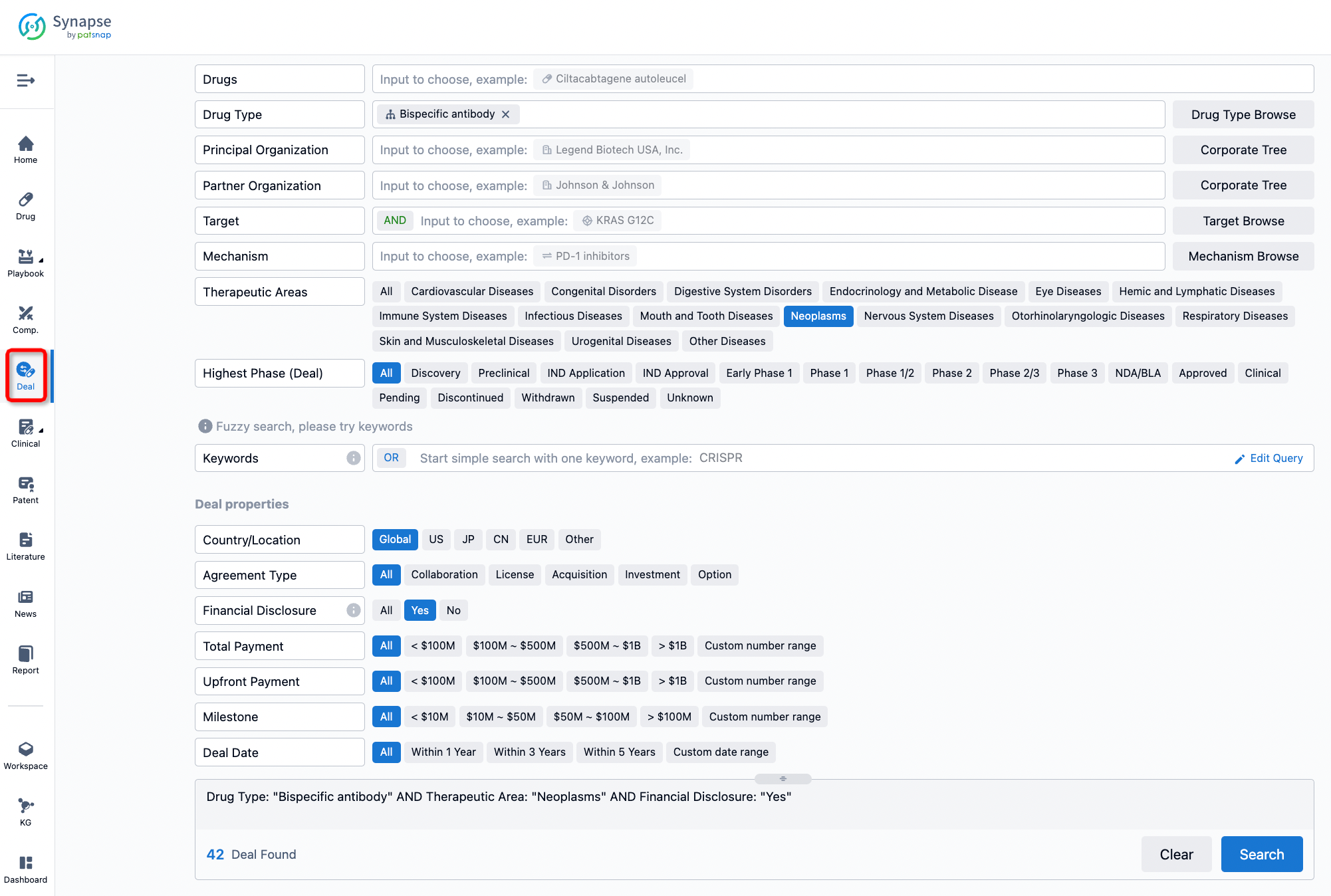
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
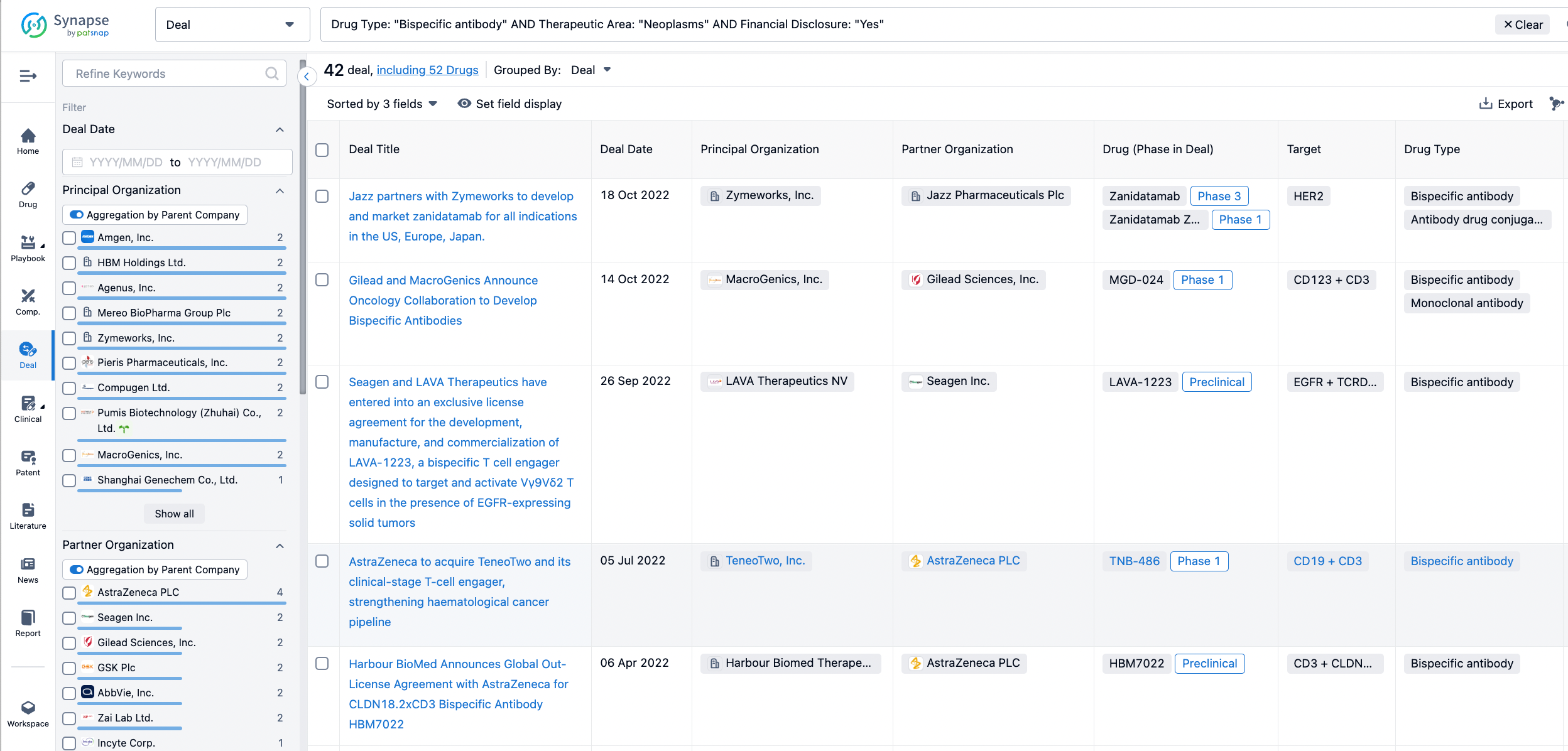
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
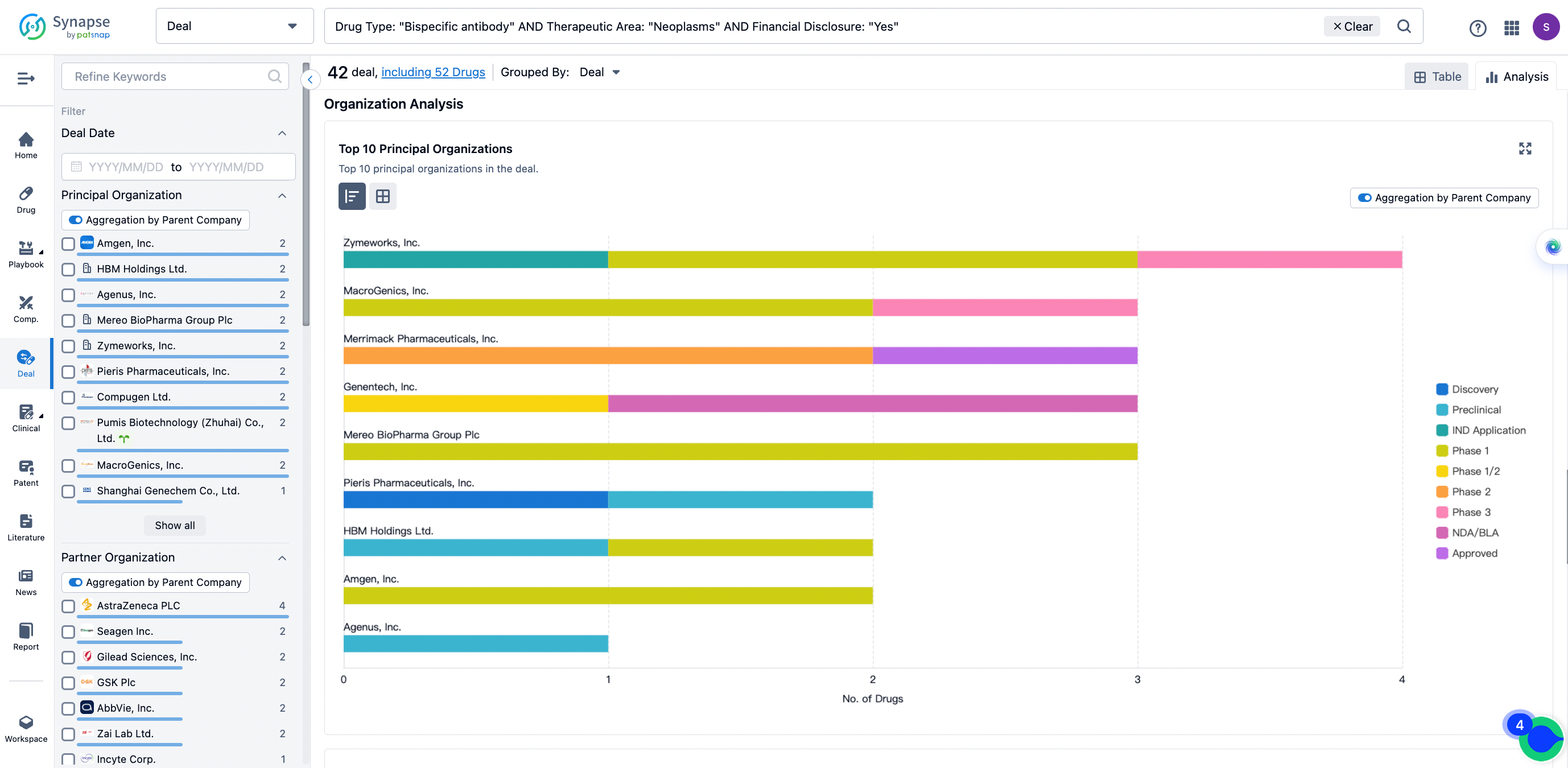
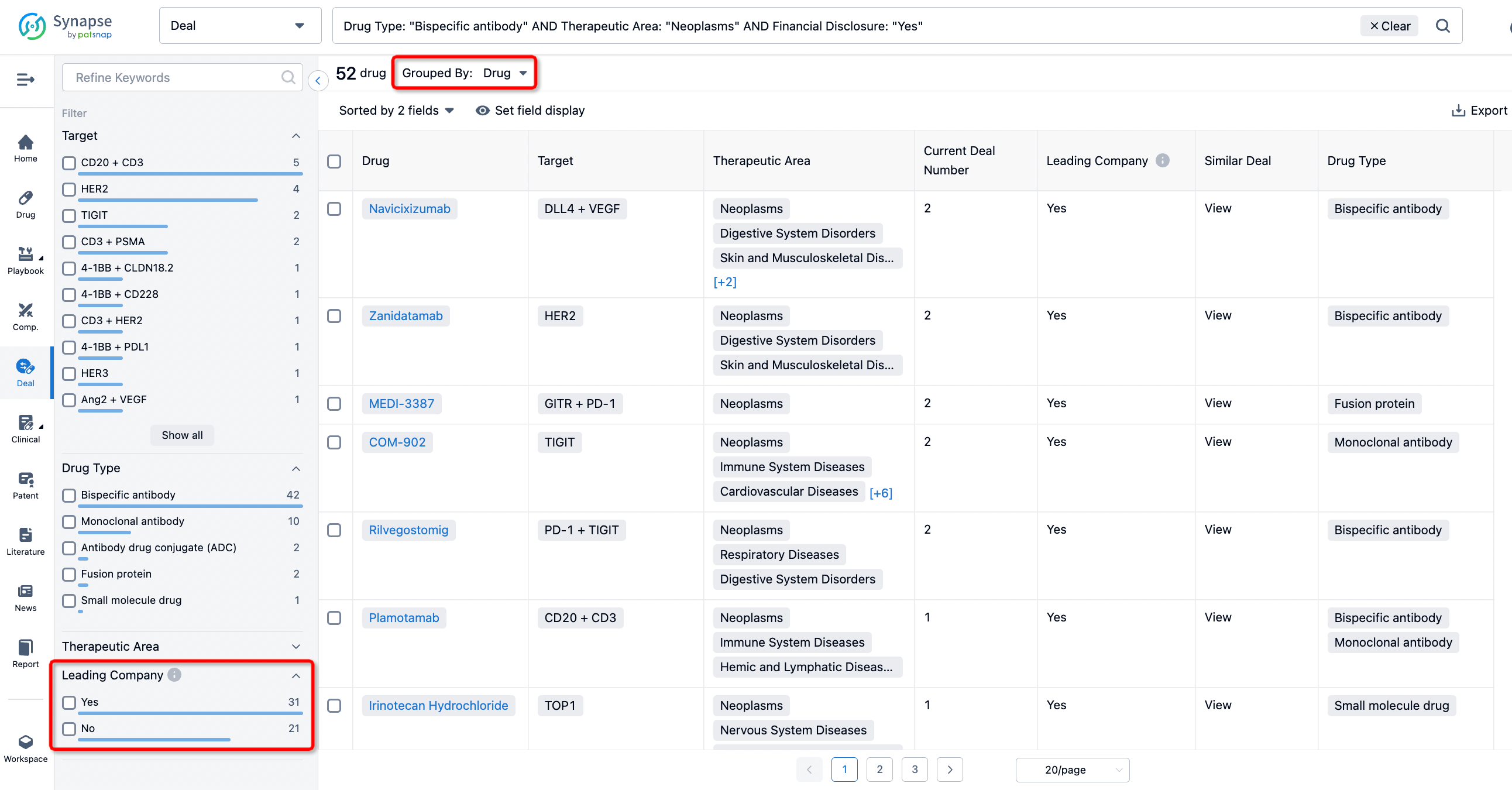
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
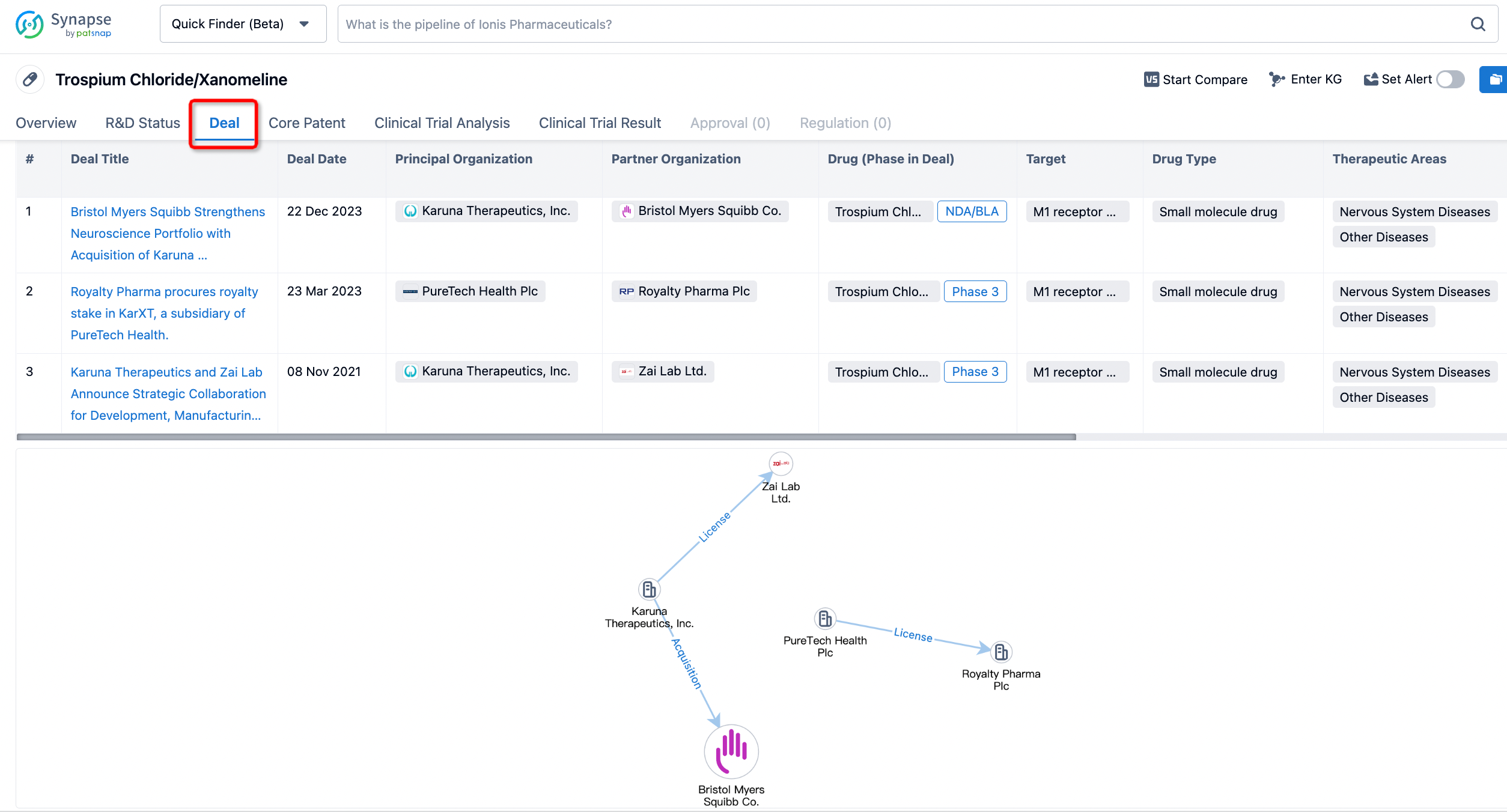
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
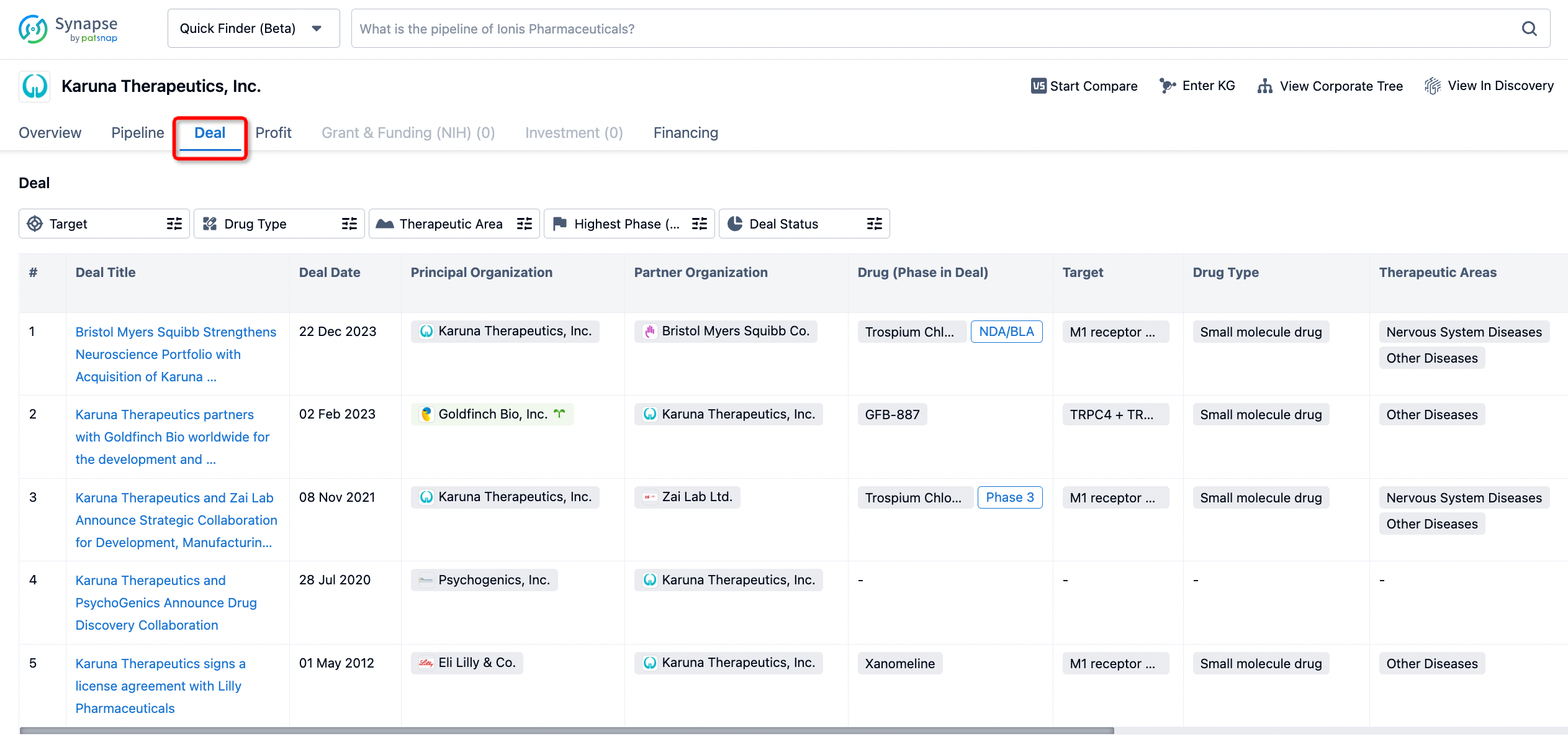
Click on the image below to embark on a brand new journey of drug discovery!