Exploring the Latest BAFF/APRIL antagonist therapy Deal by Alpine Immune Sciences: A Guide to Rapidly Accessing Transaction Insights
On April 10, 2024, Vertex Pharmaceuticals ("Vertex") and Alpine Immune Sciences ("Alpine") announced that the parties entered into a definitive agreement. the parties have entered into a definitive agreement. Pursuant to the agreement, Vertex will acquire Alpine for $65 per share, or approximately $4.9 billion in cash, a transaction that was unanimously approved by the boards of directors of Vertex and Alpine and is expected to close later this quarter.
The product focus of the acquisition is a self-immune drug in development, povtacicept (ALPN-303), which is expected to enter Phase III clinical development in the second half of 2024.
About povtacicept
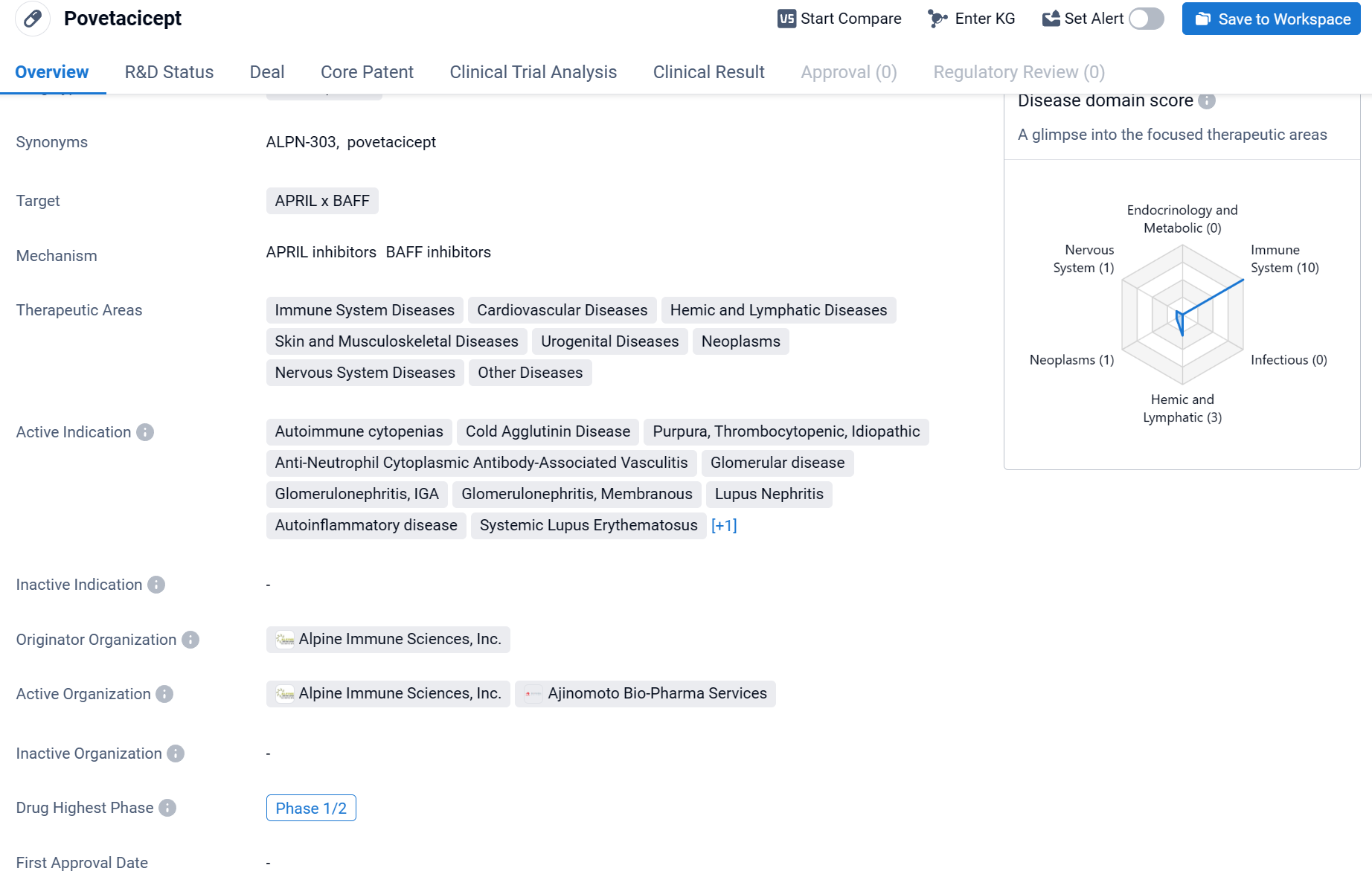
Povetacicept is a fusion protein drug that targets APRIL x BAFF, and it is being developed by Alpine Immune Sciences, Inc. The drug falls under the therapeutic areas of immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, urogenital diseases, neoplasms, nervous system diseases, and other diseases. Click the image below to directly embark on the exploration journey with the Povetacicept!
On the same day as the official announcement of the merger, the Company presented updated clinical data for povetacicept: in a multi-dose, multi-cohort, open-label, Phase 1b/2a study of povetacicept for the treatment of autoimmune glomerulonephritis, including IgA nephropathy, povetacicept was administered subcutaneously every 4 weeks; as of March 1, 2024, a total of 41 IgAN patients received subcutaneous injections of 80 or 240 mg of povetacicept every 4 weeks and showed that: treatment with povetacicept 80 mg SC Q4W had a clinically meaningful improvement in proteinuria, with a 64.1% reduction in the urinary protein-to-creatinine ratio (UPCR; n=6) from baseline at 36 weeks; and at 36 weeks, a significant reduction in proteinuria as assessed by glomerular filtration rate ( eGFR) assessed at 36 weeks to stabilize renal function.
About Alpine Immune Sciences
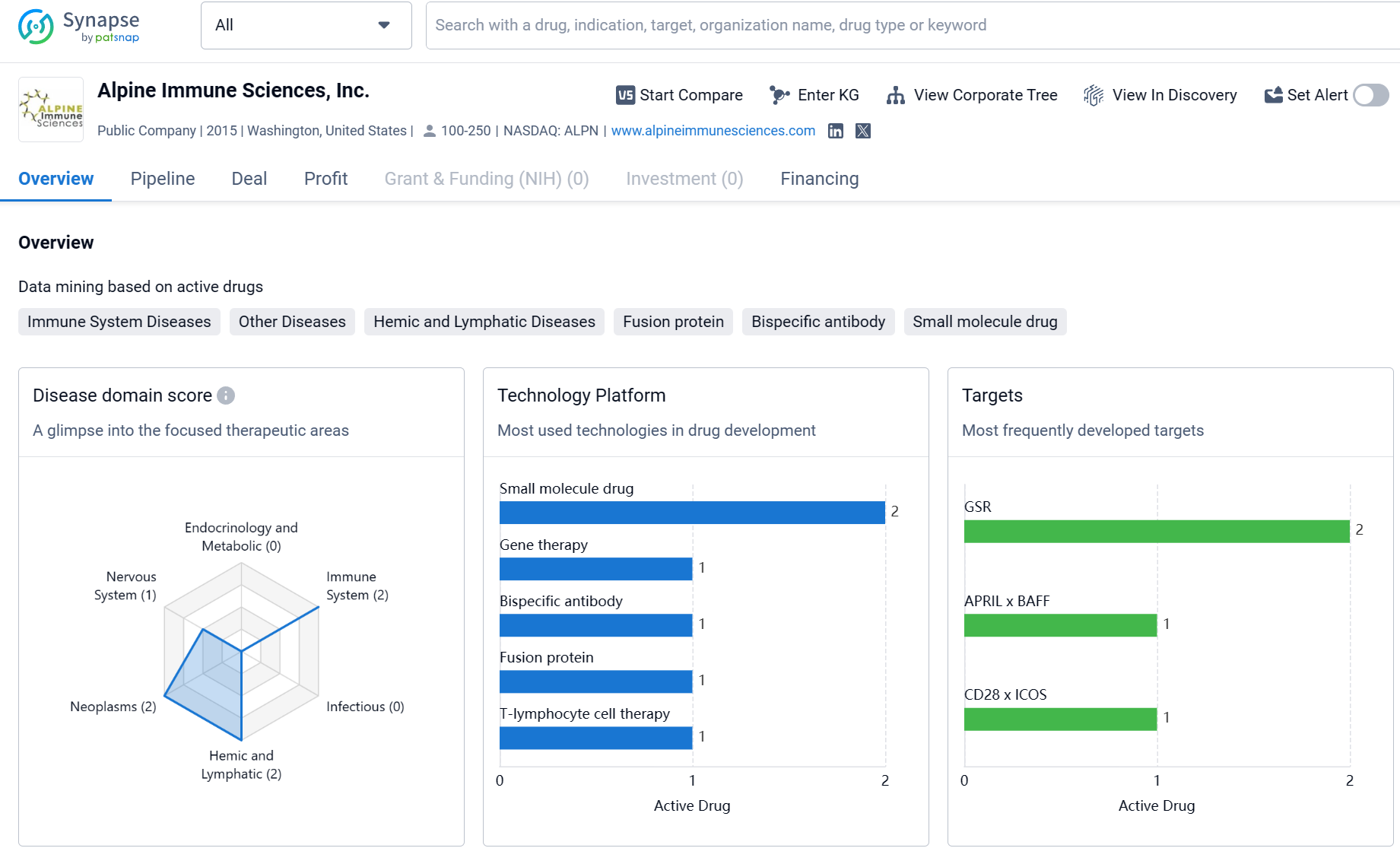
Alpine Immune Sciences, Inc. is actively engaged in the development of innovative therapies in the field of biomedicine. The company's diverse portfolio of drugs and focus on specific targets indicate a commitment to addressing unmet medical needs in various therapeutic areas. As the pipeline progresses, it will be interesting to see the outcomes of Alpine Immune Sciences, Inc.'s research and development efforts and the potential impact of their therapies on patients' lives.
How to get the latest progress on drug deals?
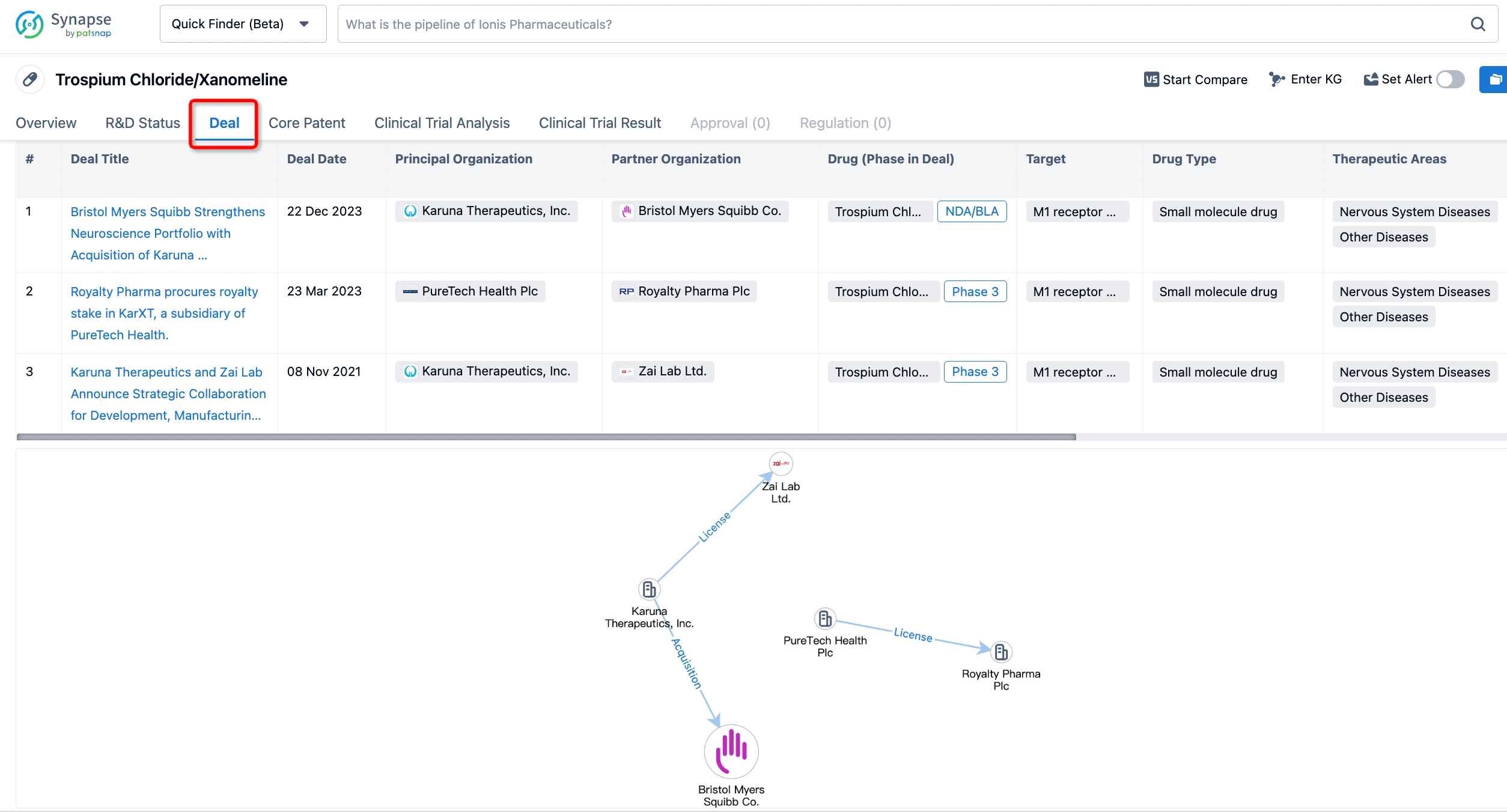
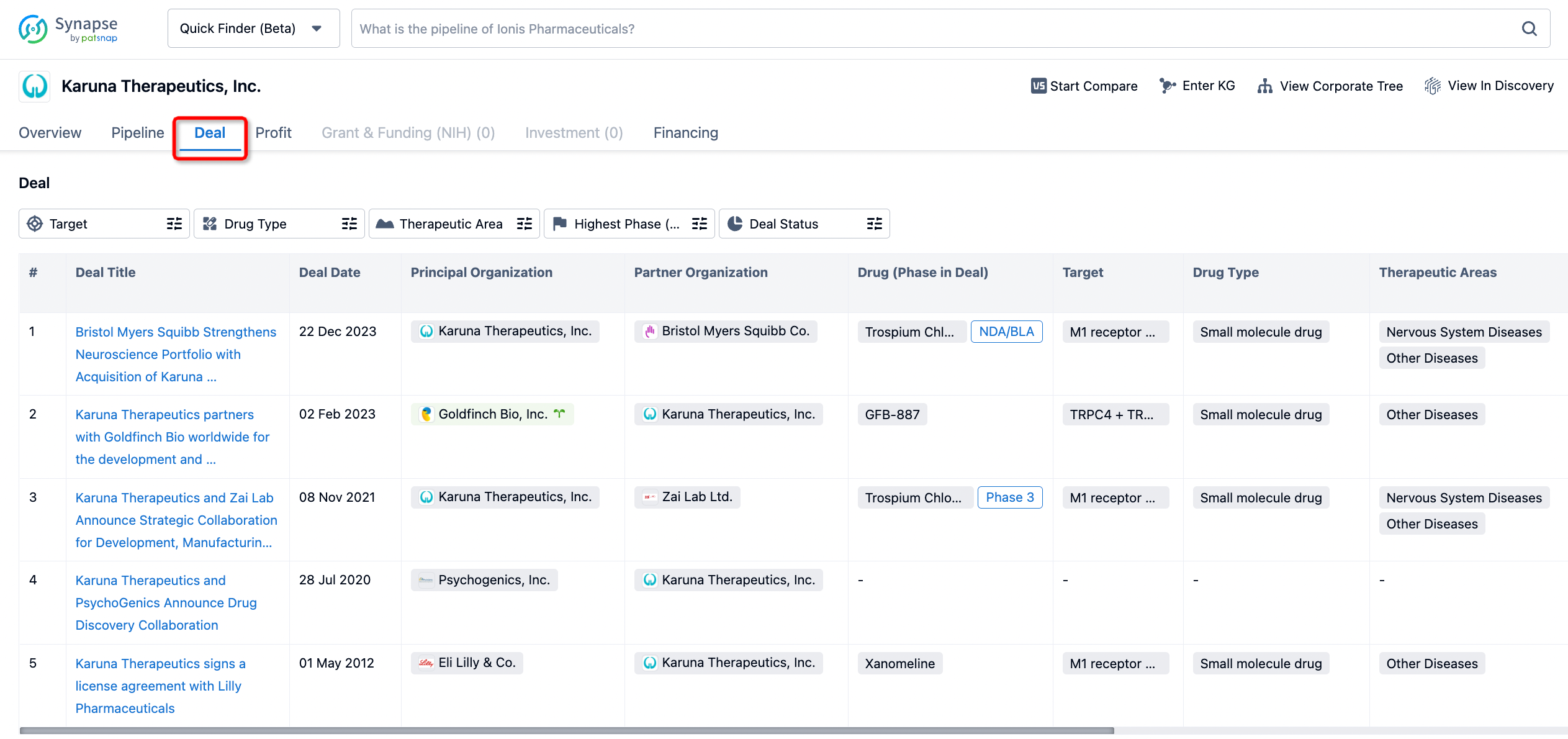
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
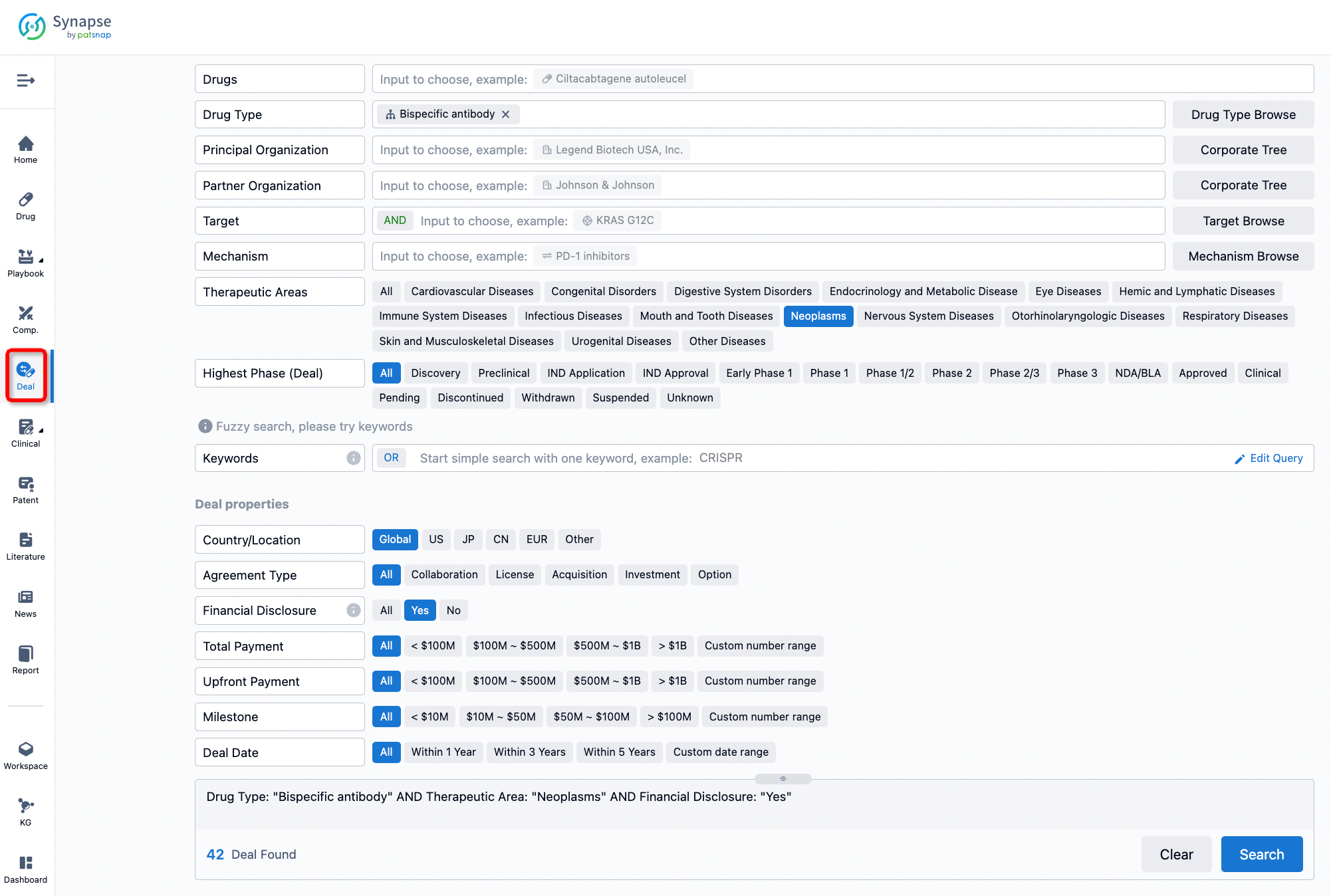
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
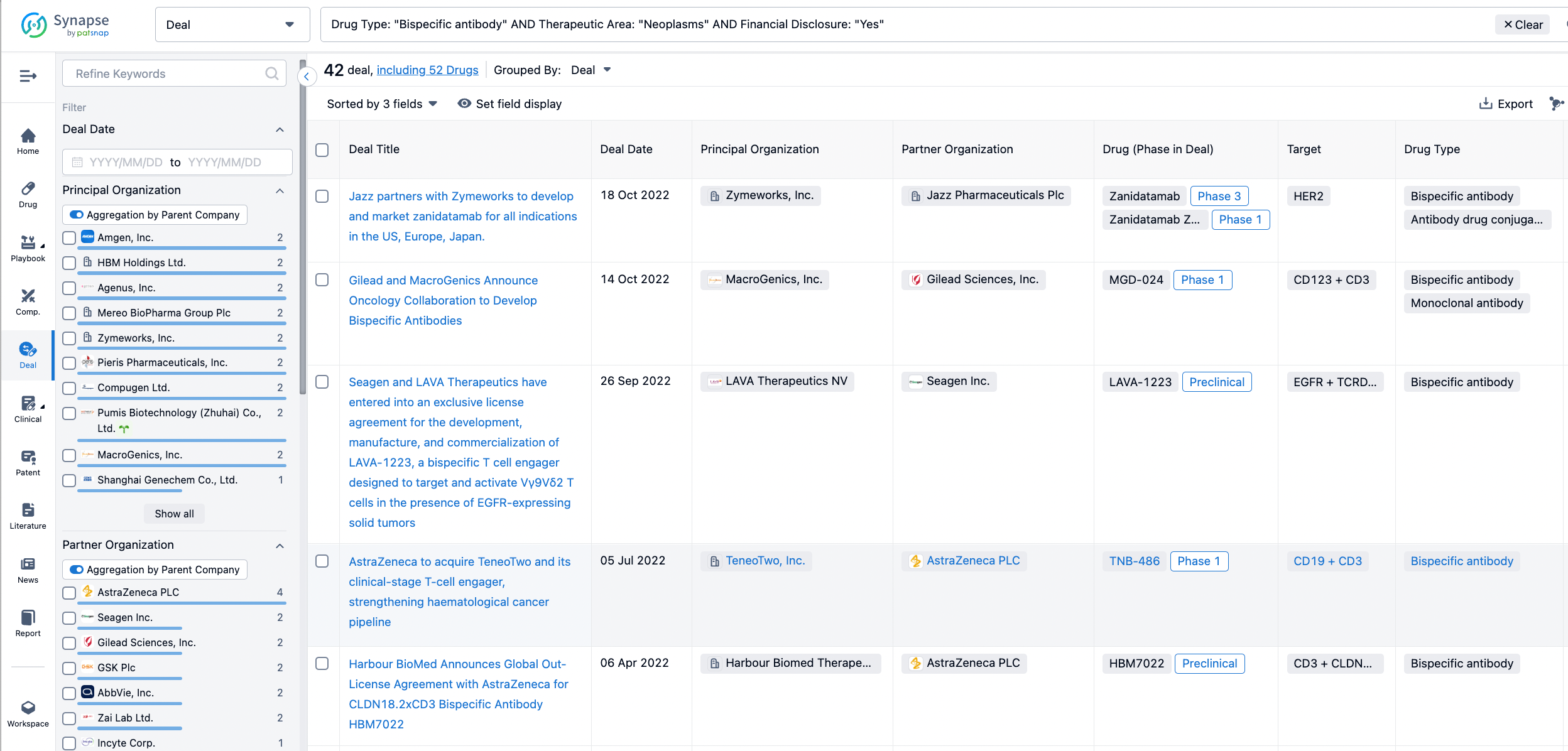
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
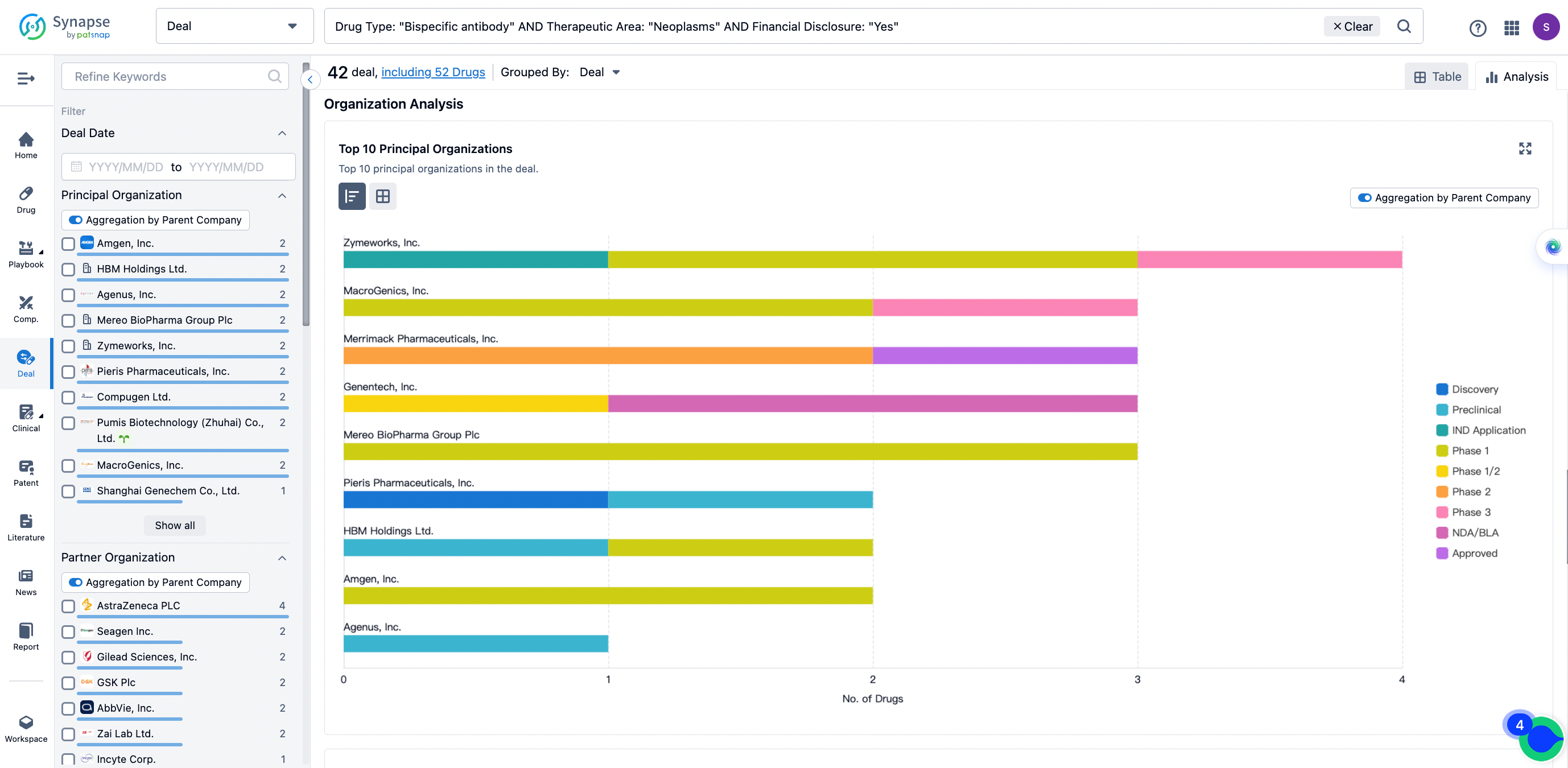
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
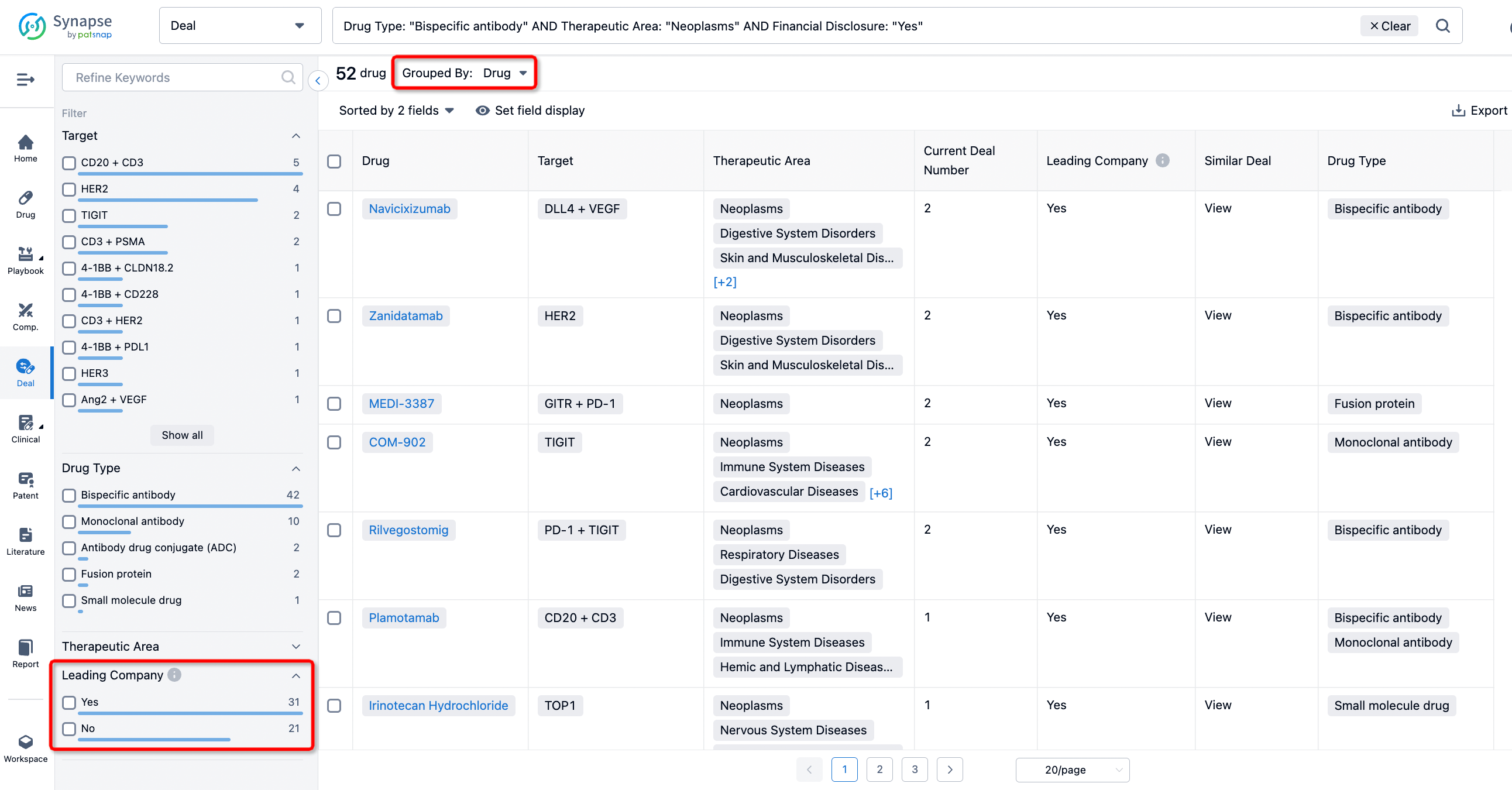
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to embark on a brand new journey of drug discovery!