Exploring the Latest New Endocrine Therapy for ESR1 Mutation Breast Cancer by Shanghai Henlius Biotech: A Guide to Rapidly Accessing Transaction Insights
On January 11, 2024, Shanghai Henlius Biotech, Inc. and Sermonix Pharmaceuticals LLC jointly announced that they have entered into a strategic collaboration and exclusive licensing agreement to develop, produce, and commercialize Sermonix's key investigational product, lasofoxifene, in China. Under the terms of the agreement, Henlius has been granted the exclusive license to develop, produce, and commercialize lasofoxifene in China, while Sermonix retains rights in other global regions; Henlius will join the international multicenter Phase III clinical study of lasofoxifene and will be responsible for the clinical development within the cooperative region; Sermonix will receive an upfront payment, milestone payments of up to $58 million, and a share of the sales revenue in the cooperative region.
About Lasofoxifene
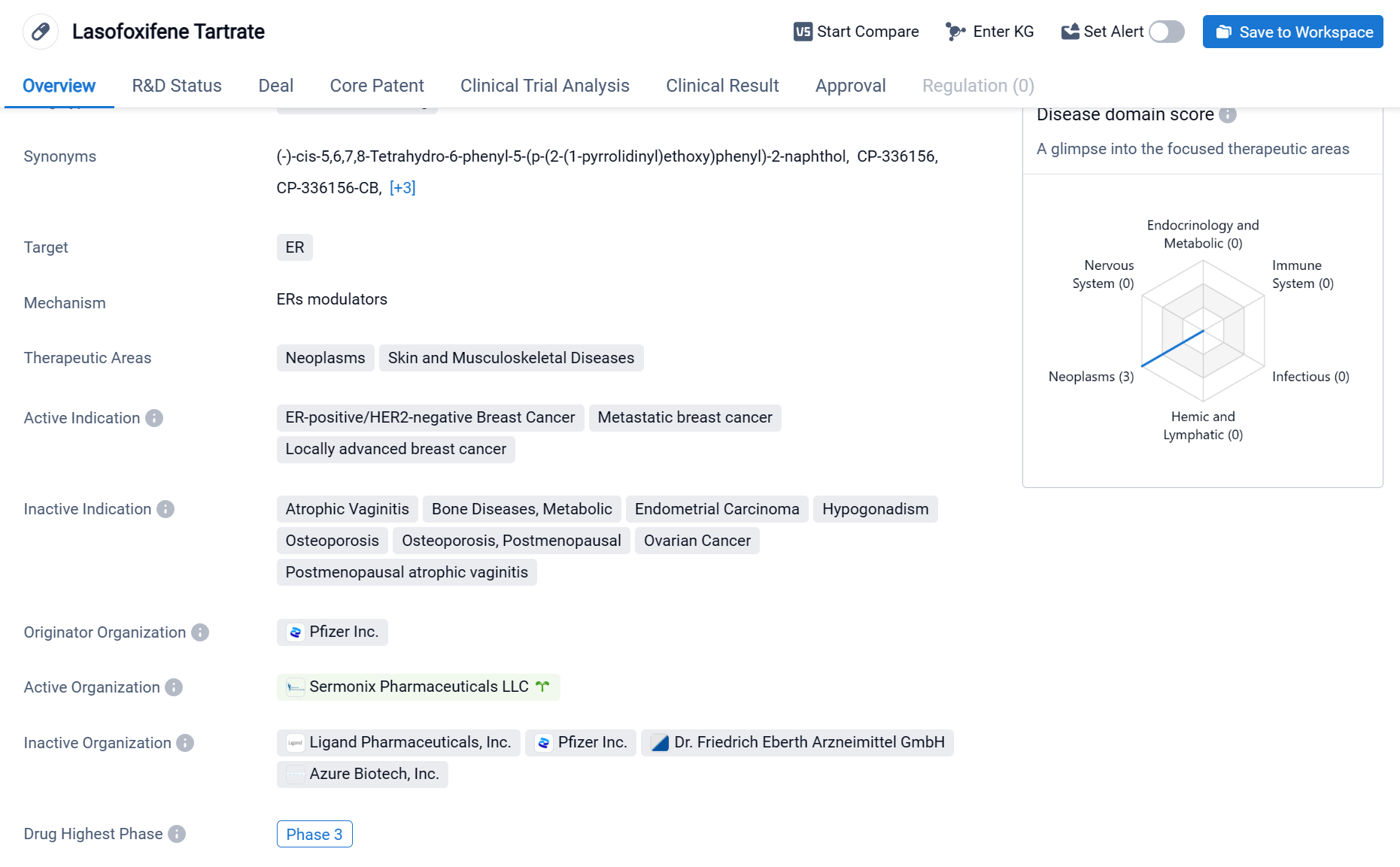
Lasofoxifene is a small molecule drug that targets the estrogen receptor (ER) and is used in the treatment of various neoplasms, skin diseases, and musculoskeletal diseases. It is primarily indicated for ER-positive/HER2-negative breast cancer, metastatic breast cancer, and locally advanced breast cancer. The drug was developed by Pfizer Inc., a renowned pharmaceutical company. It has reached the highest phase of clinical development, which is Phase 3, on a global scale. Click the image below to directly embark on the exploration journey with the lasofoxifene!
The phase II clinical studies have shown that lasofoxifene, whether used as a monotherapy or in combination with CDK4/6 inhibitors, exhibits antitumor activity. Its unique tissue selectivity sets it apart from other existing and investigational endocrine therapies, and can complement and enhance the strengths of Fosun Pharma's existing breast cancer product pipeline. Furthermore, past clinical studies have proven that it can also have beneficial effects on the vagina and bones.
In terms of ESR1 mutation tumors, the company has completed two Phase II studies (ELAINE-1 and ELAINE-2 studies) in which lasofoxifene demonstrated significant clinical efficacy. The ELAINE-3 study is a Phase III trial that evaluates lasofoxifene in a patient population with ESR1 mutations, investigating the efficacy of lasofoxifene in combination with Eli Lilly's CDK4/6 inhibitor abemaciclib (Verzenio®), compared to fulvestrant with abemaciclib, in 400 premenopausal and postmenopausal participants with locally advanced or metastatic ER+/HER2- breast cancer harboring ESR1 mutations.
About Shanghai Henlius Biotech, Inc.
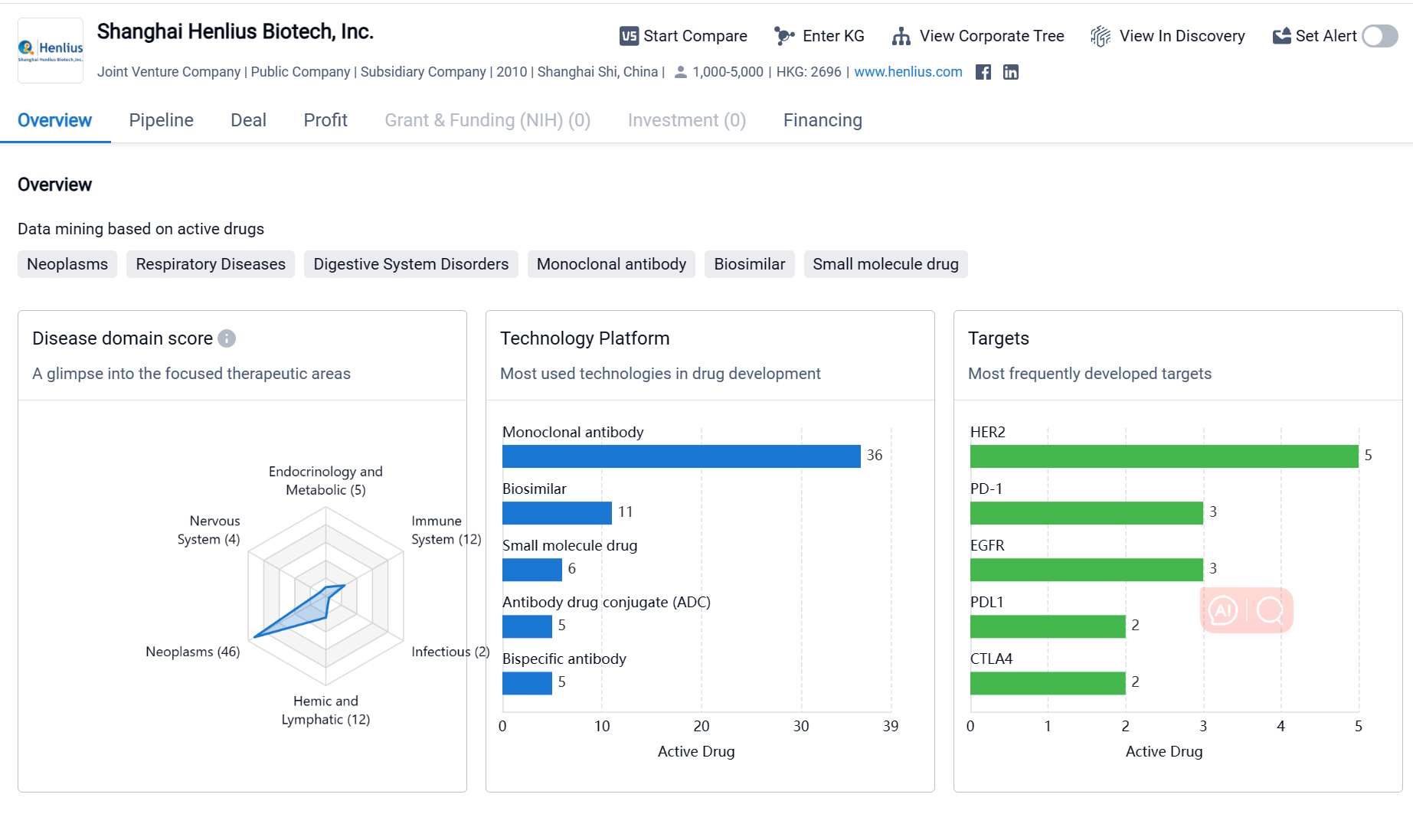
Shanghai Henlius Biotech, Inc. is a biopharmaceutical company based in Shanghai, China. It was founded in 2010 and has since become a prominent player in the pharmaceutical industry. The company focuses on the development and commercialization of innovative biologic drugs for the treatment of various diseases. Shanghai Henlius Biotech has a diverse portfolio of drugs targeting different therapeutic areas, with a particular emphasis on neoplasms. The company has also developed drugs targeting various proteins and receptors involved in disease progression. Its pipeline includes drugs at different stages of development, indicating active engagement in drug development and potential for future growth.
How to get the latest progress on drug deals?
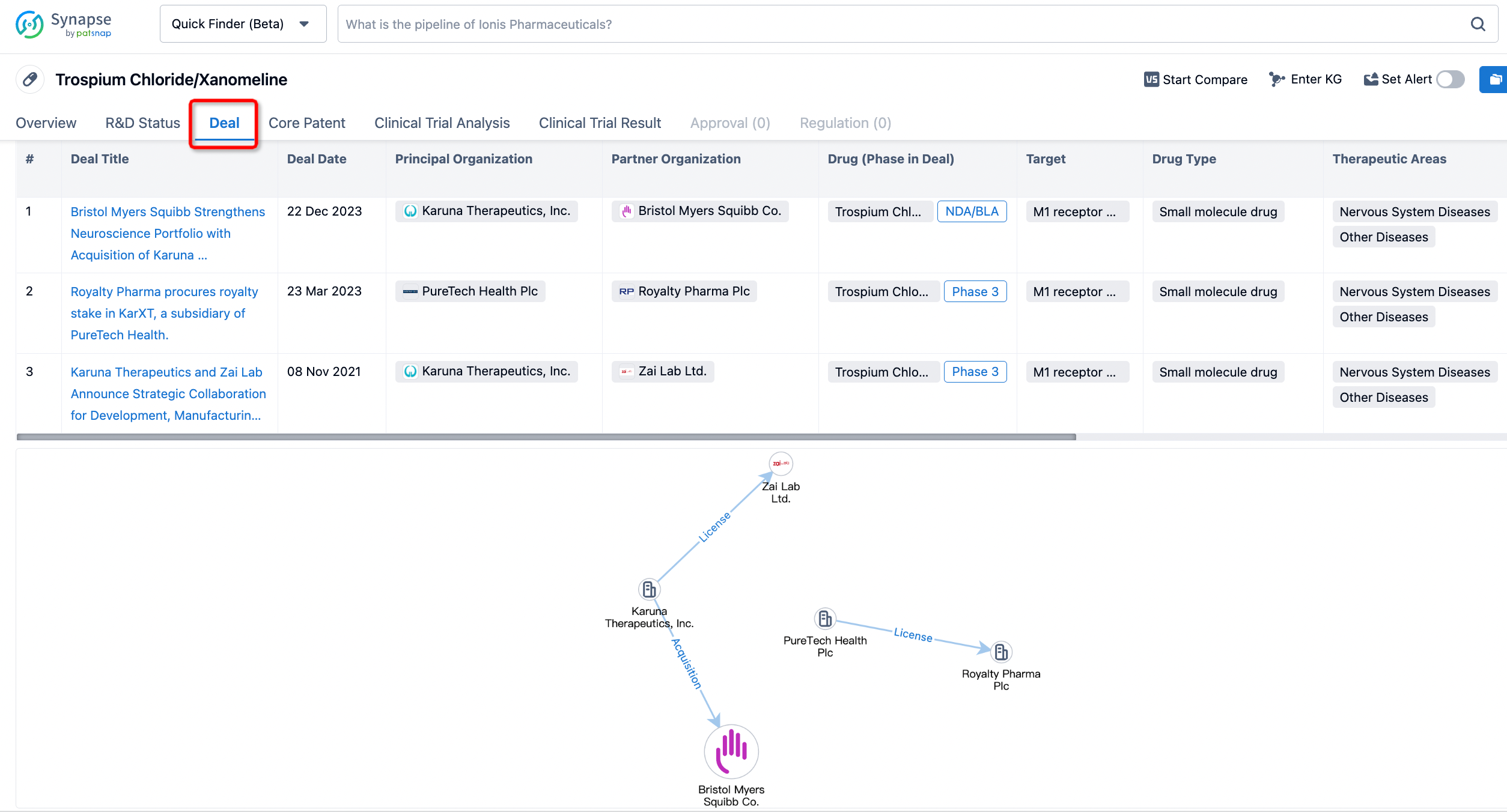
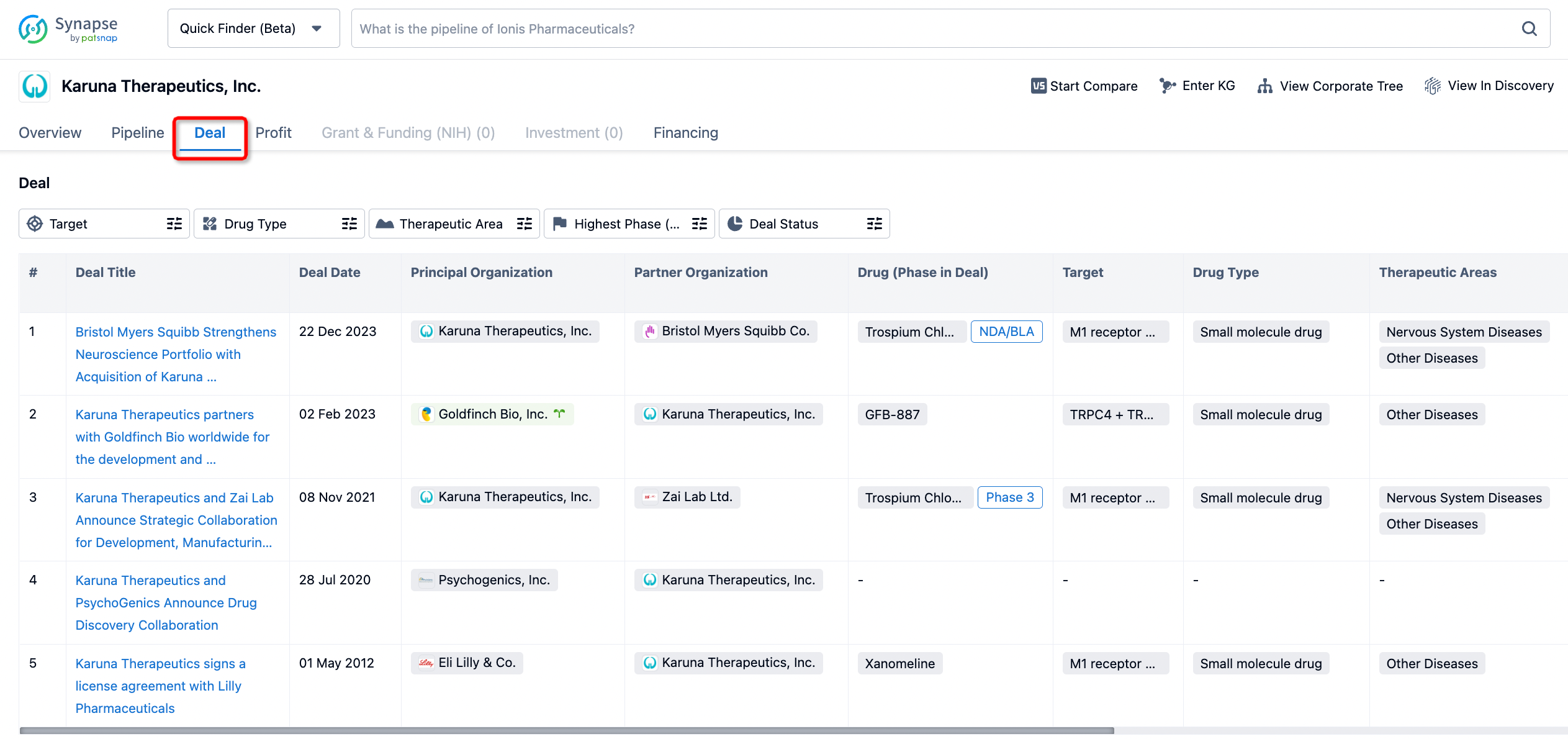
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
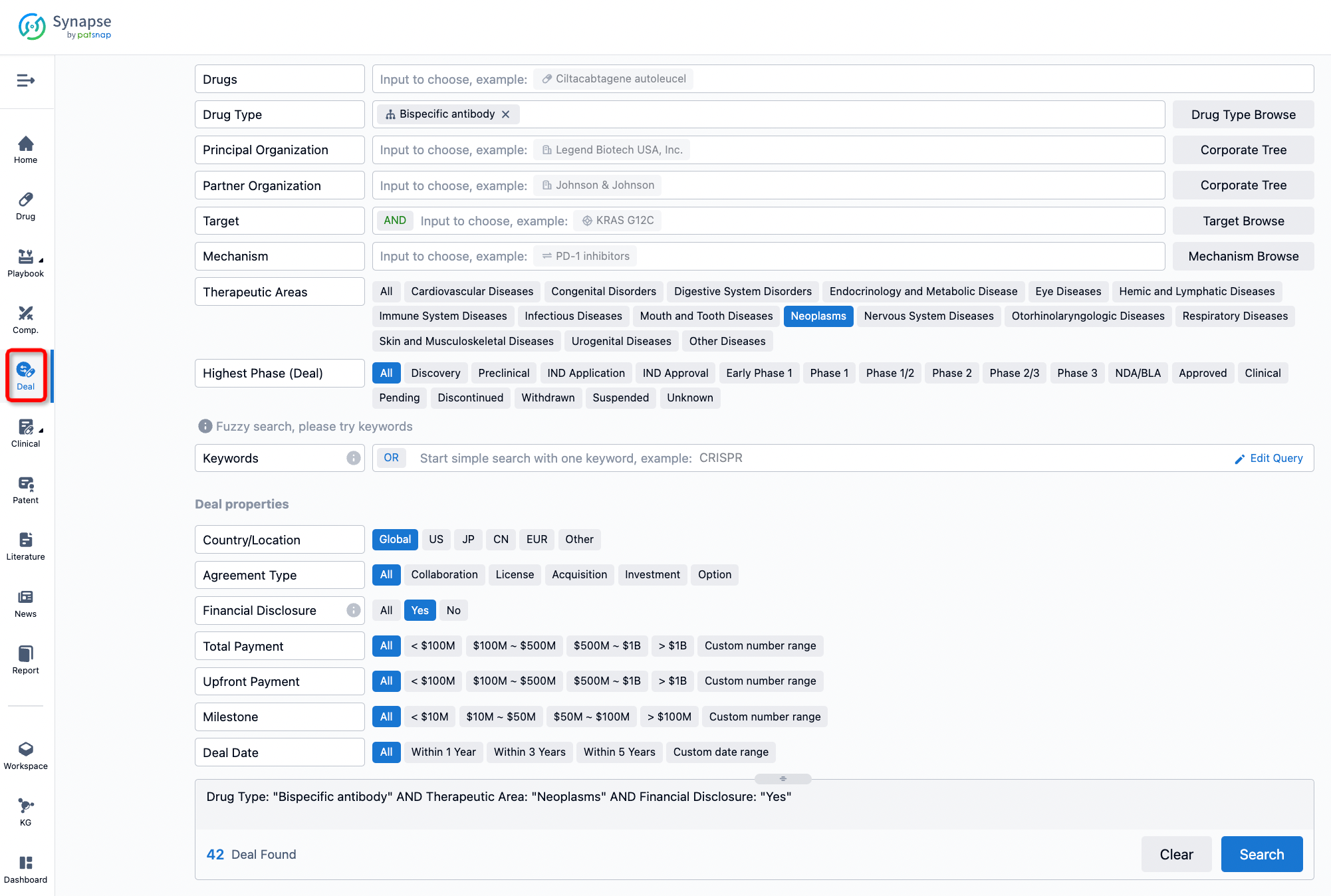
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
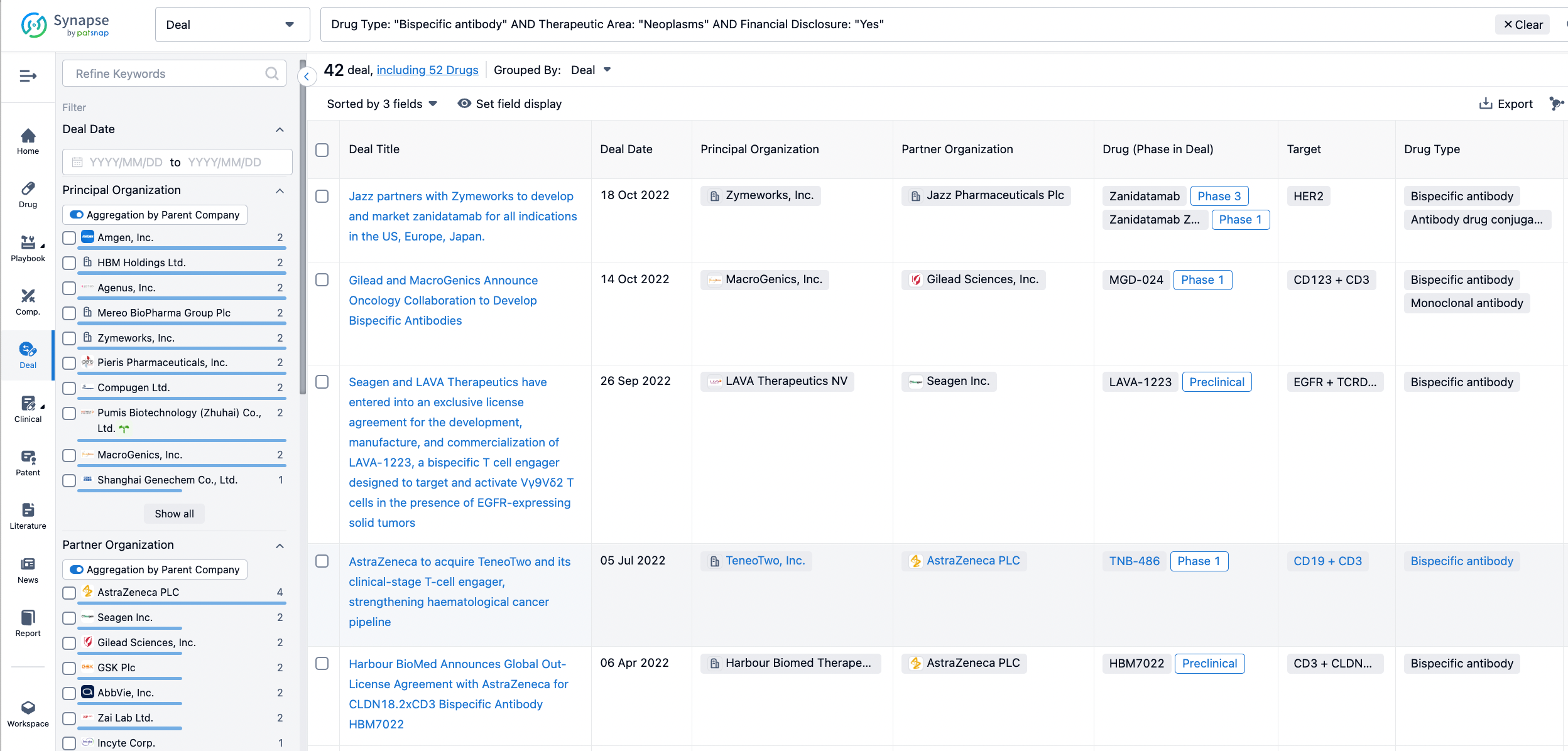
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
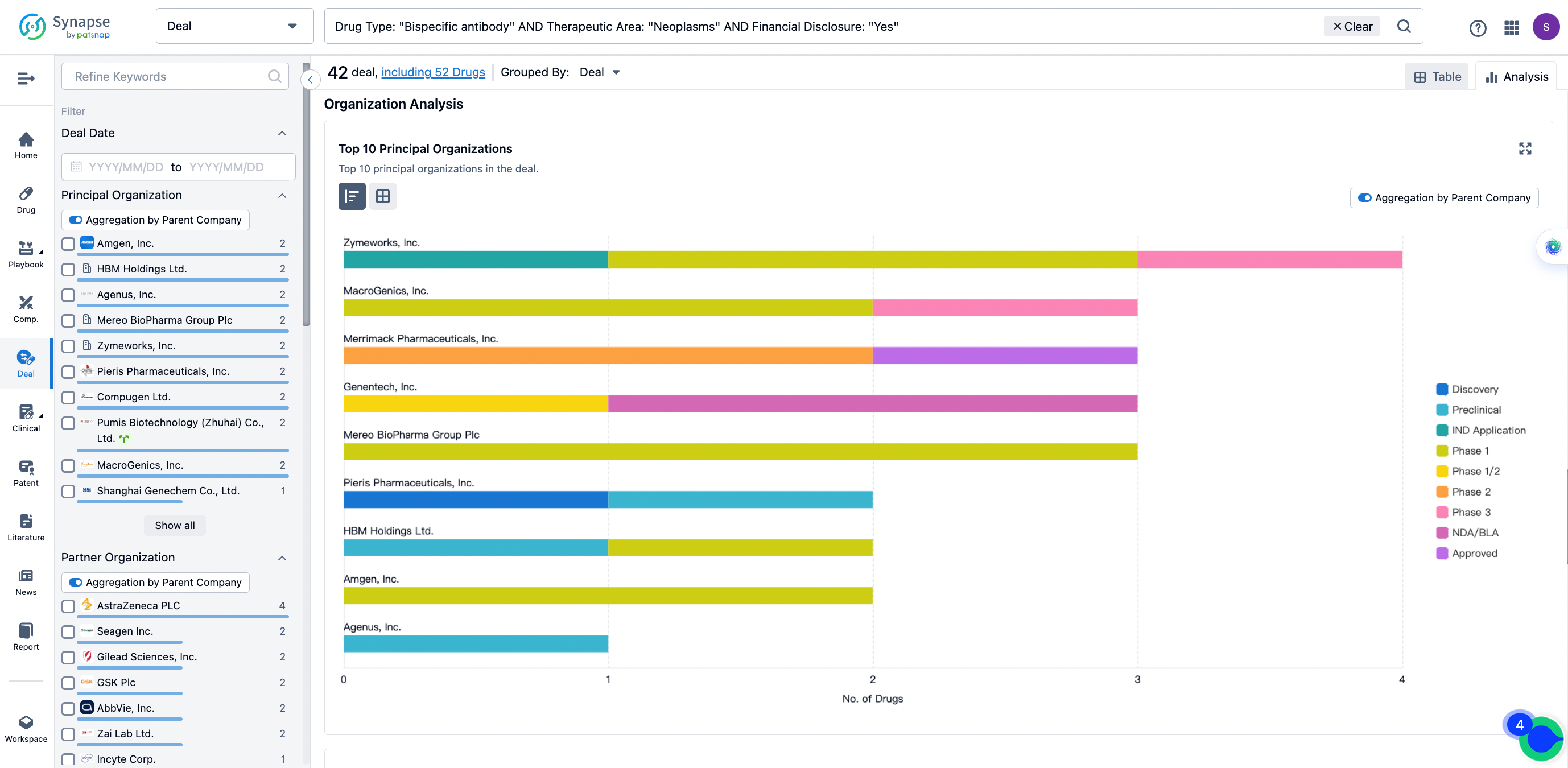
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
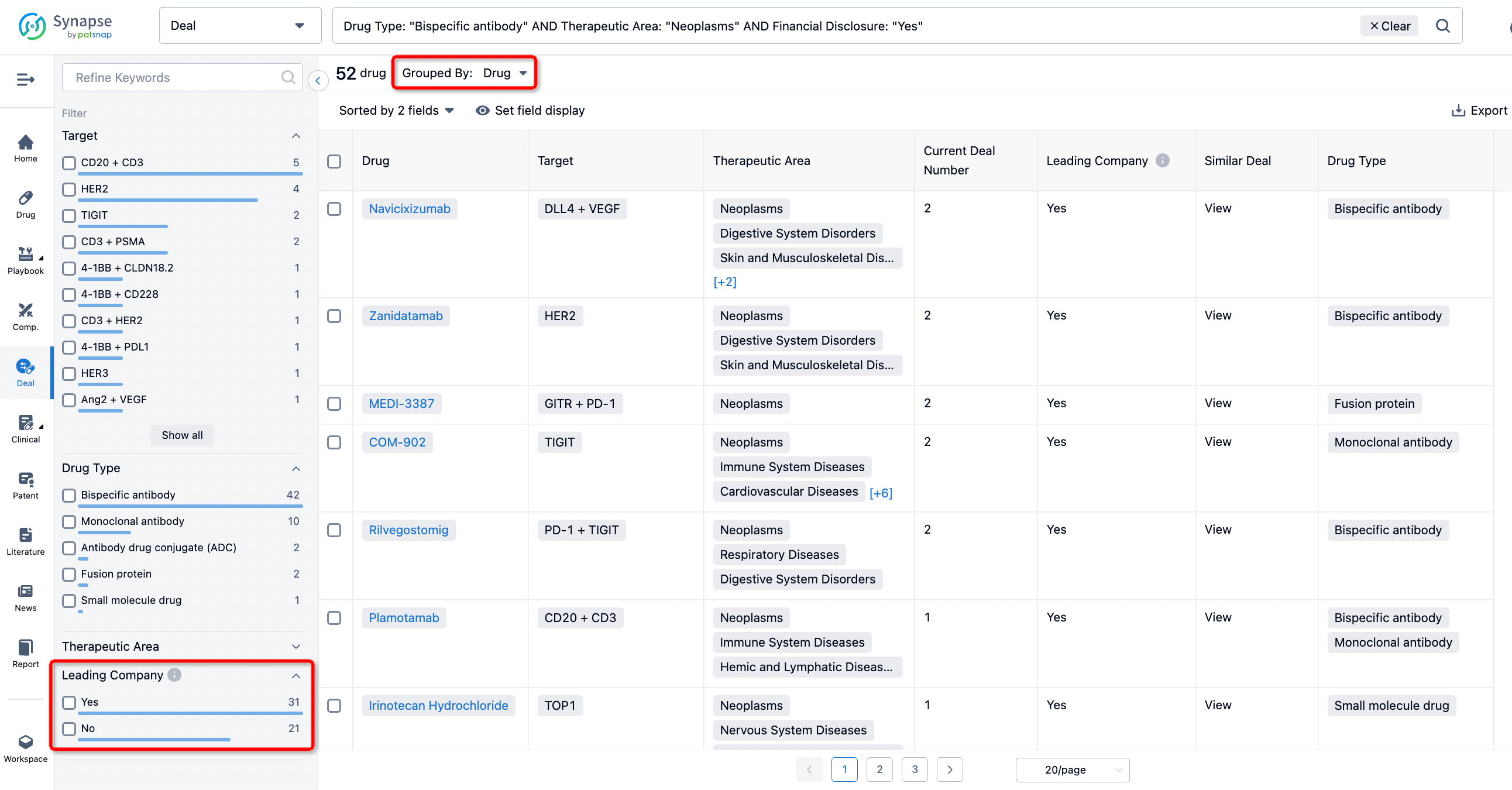
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to embark on a brand new journey of drug discovery!