Exploring the Latest PROTACs Deal by Kymera Therapeutics: A Guide to Rapidly Accessing Transaction Insights
On December 7, 2023, Kymera Therapeutics, Inc. announced that the first patient had been dosed in the Phase 2 ADVANTA study to evaluate the effect of KT-474 (SAR444656) in atopic dermatitis (AD), triggering a $15 million milestone payment from the collaboration with Sanofi. In July 2020, Kymera had entered into a strategic collaboration with Sanofi for multiple programs, securing an upfront payment of $150 million and the potential for over $2 billion in developmental, regulatory, and sales milestones, along with significant royalty payments. Sanofi committed to jointly advancing KT-474 into Phase 2 clinical trials.
About KT-474
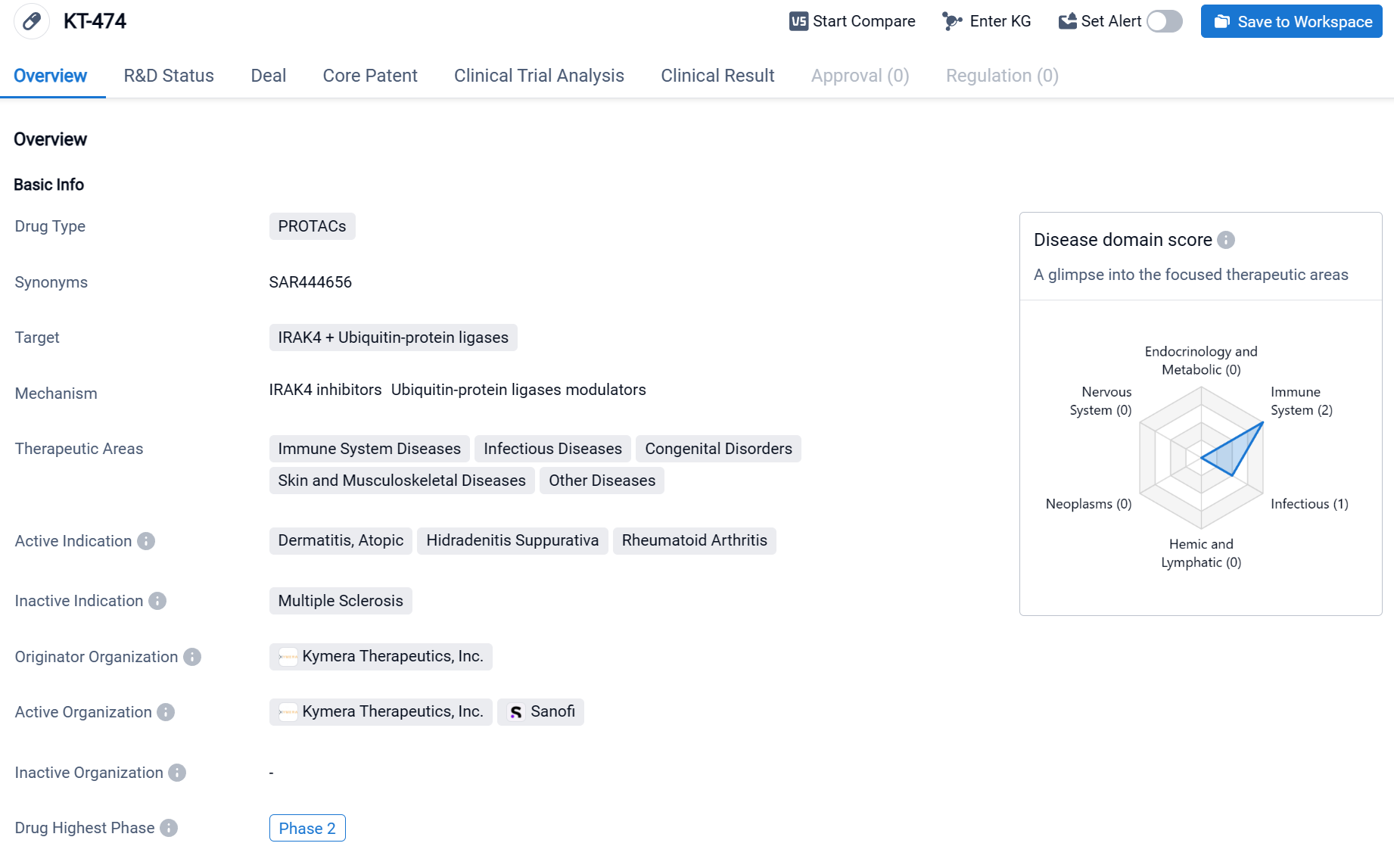
KT-474 is a drug classified as PROTACs, which stands for Proteolysis Targeting Chimeras. This type of drug works by utilizing small molecules to target specific proteins for degradation within cells. In the case of KT-474, it targets two proteins: IRAK4 and Ubiquitin-protein ligases. The therapeutic areas that KT-474 focuses on include immune system diseases, infectious diseases, congenital disorders, skin and musculoskeletal diseases, as well as other diseases. This suggests that the drug has a broad potential for treating various conditions related to these areas. Click the image below to directly embark on the exploration journey with the KT-474 !
A randomized, placebo-controlled, single and multiple ascending dose trial (Phase 1) evaluated the safety, pharmacokinetics, pharmacodynamics, and clinical activity of KT-474. The study results revealed that IRAK4 degradation was observed in the blood of healthy volunteers, with an average reduction of ≥93% following single doses of 600-1600 mg, and an average reduction of ≥95% 14 days after single doses of 50-200 mg. Similar IRAK4 degradation was also achieved in the blood of patients treated with 75 mg of KT-474. In terms of safety, the drug was well-tolerated with no drug-related infections reported.
About Kymera Therapeutics, Inc.
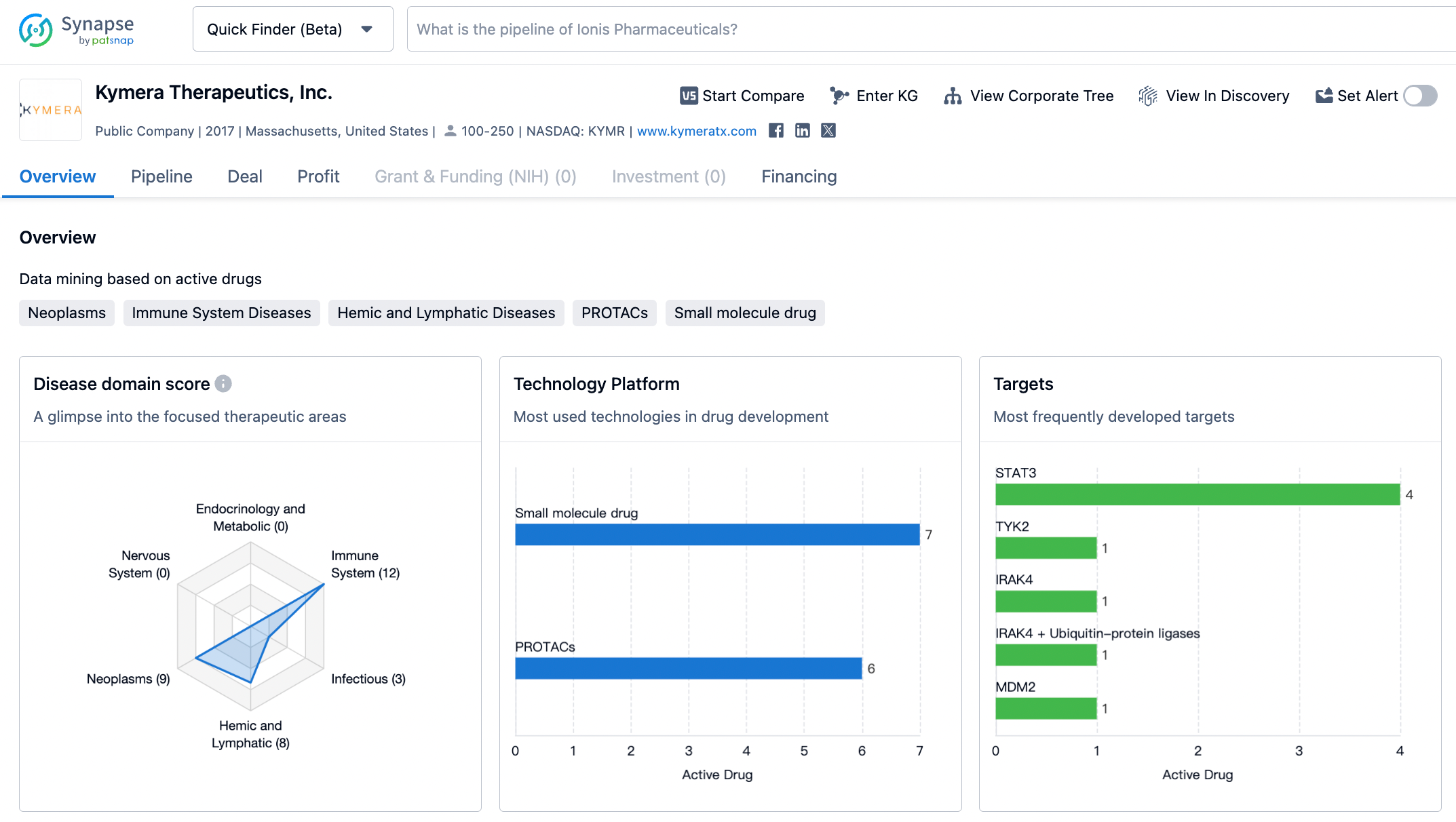
Kymera Therapeutics, Inc. is a biopharmaceutical company that was founded in 2017 and is based in Massachusetts, United States. The company focuses on developing targeted protein degradation therapies for various diseases. Kymera Therapeutics has a diverse portfolio of drugs, with a strong focus on immune system diseases and neoplasms. The company is actively developing drugs that target proteins such as STAT3, TYK2, IRAK4, MDM2, and IL-3 + IL-4. While Kymera Therapeutics has made progress in its drug development pipeline, it is still in the early stages, with drugs in the discovery and preclinical phases. The company has not yet submitted any IND applications or received IND approvals, indicating that it has not yet reached the stage of conducting human clinical trials. Overall, Kymera Therapeutics shows promise in developing targeted protein degradation therapies, but further progress is needed to advance its drug candidates through clinical development and regulatory approval.
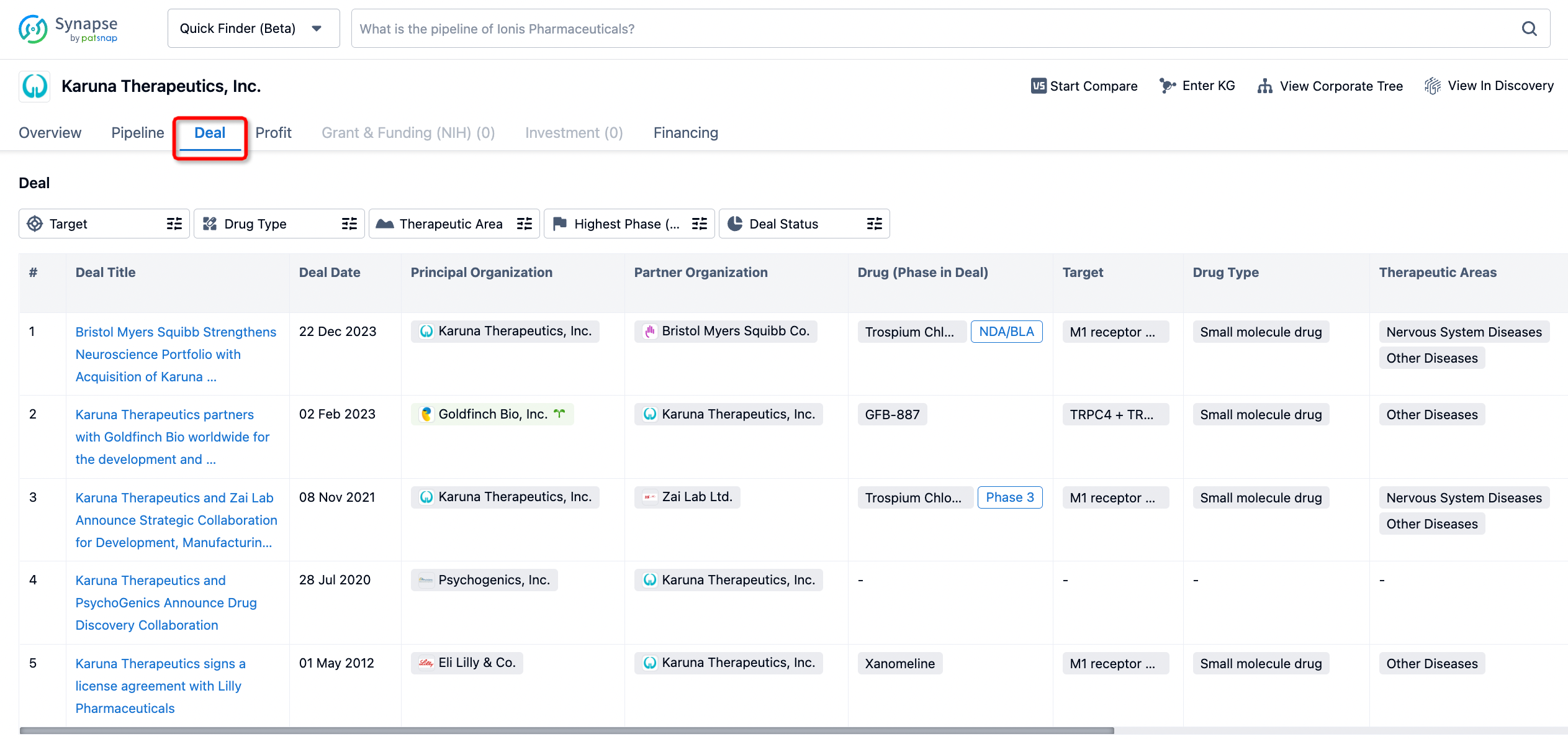
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
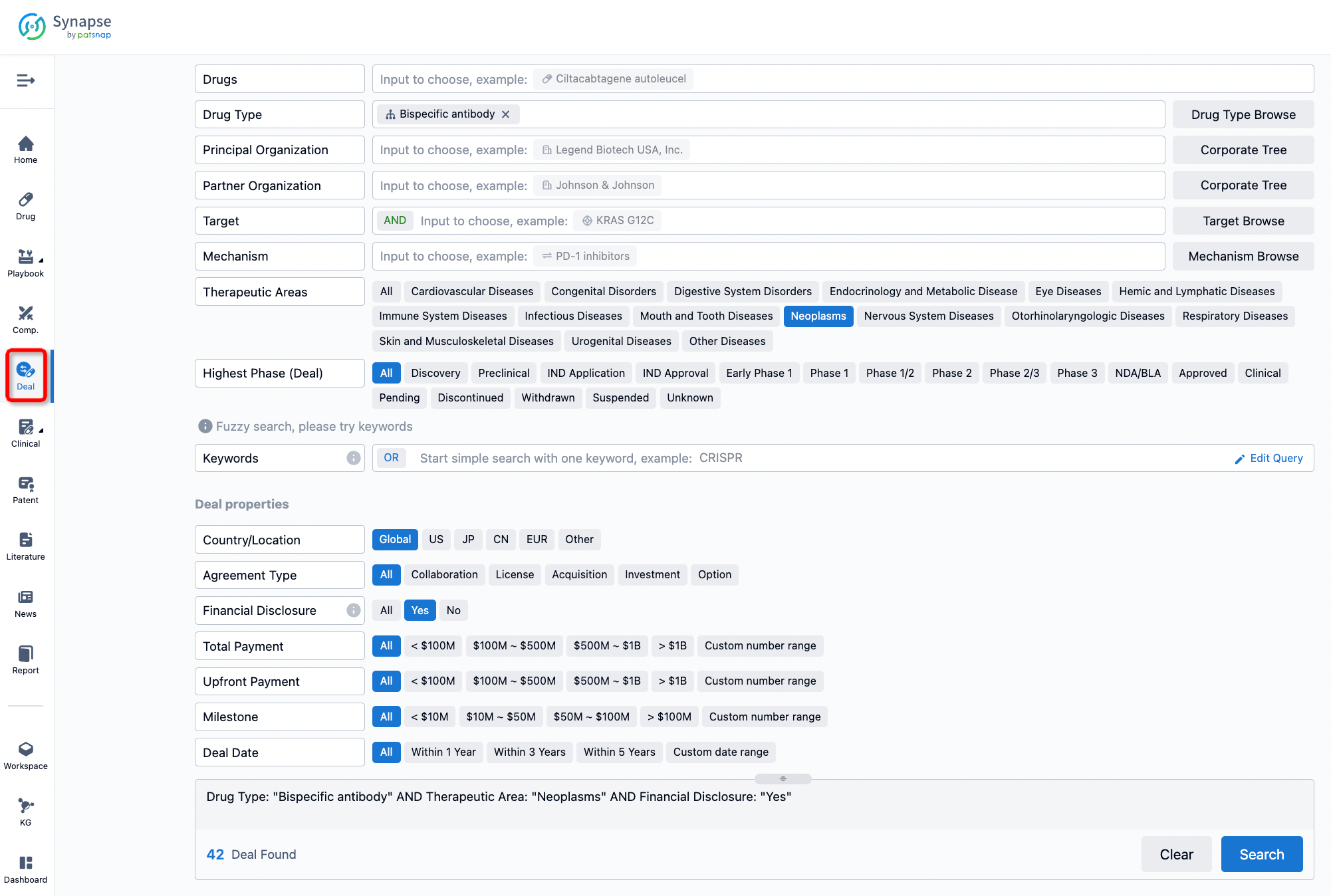
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
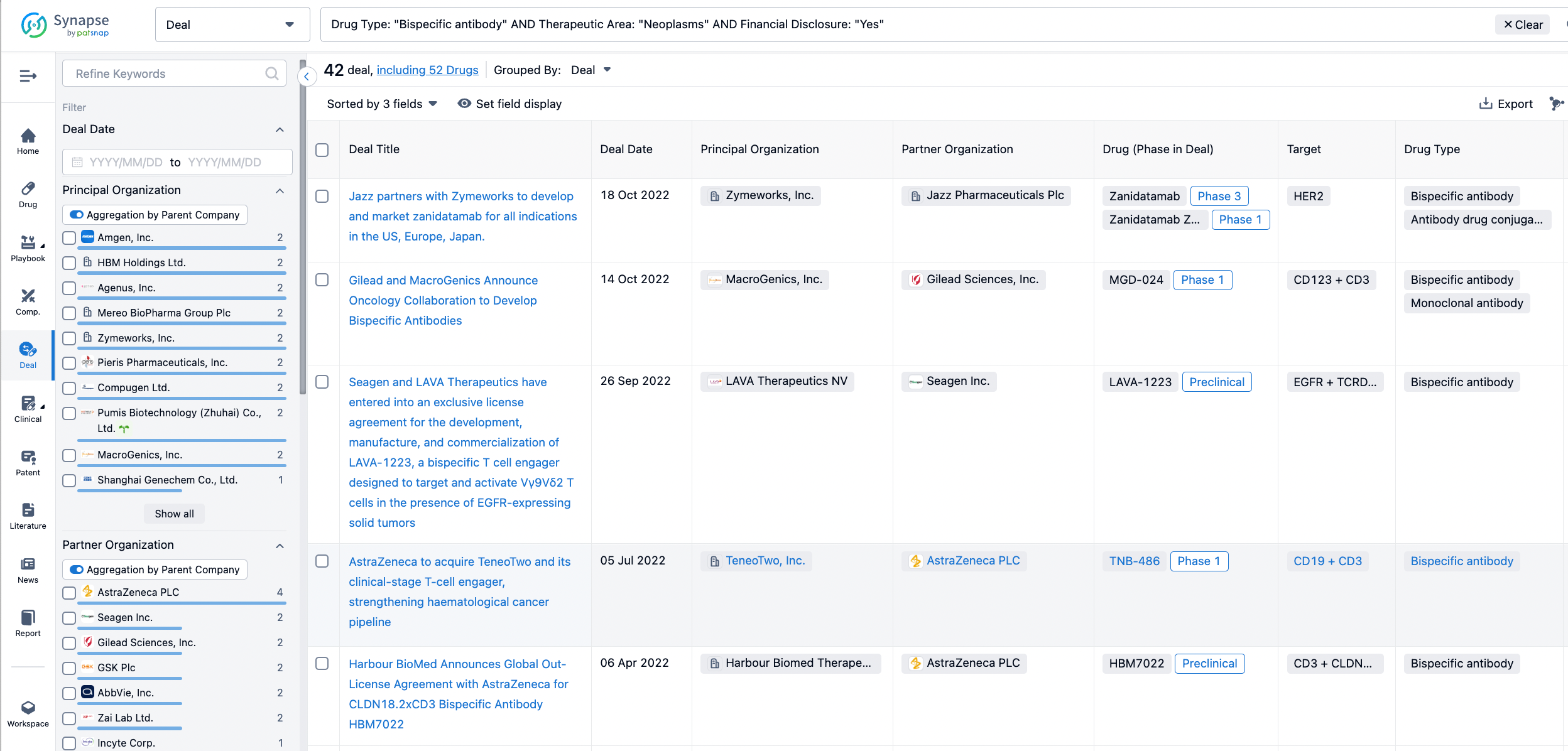
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
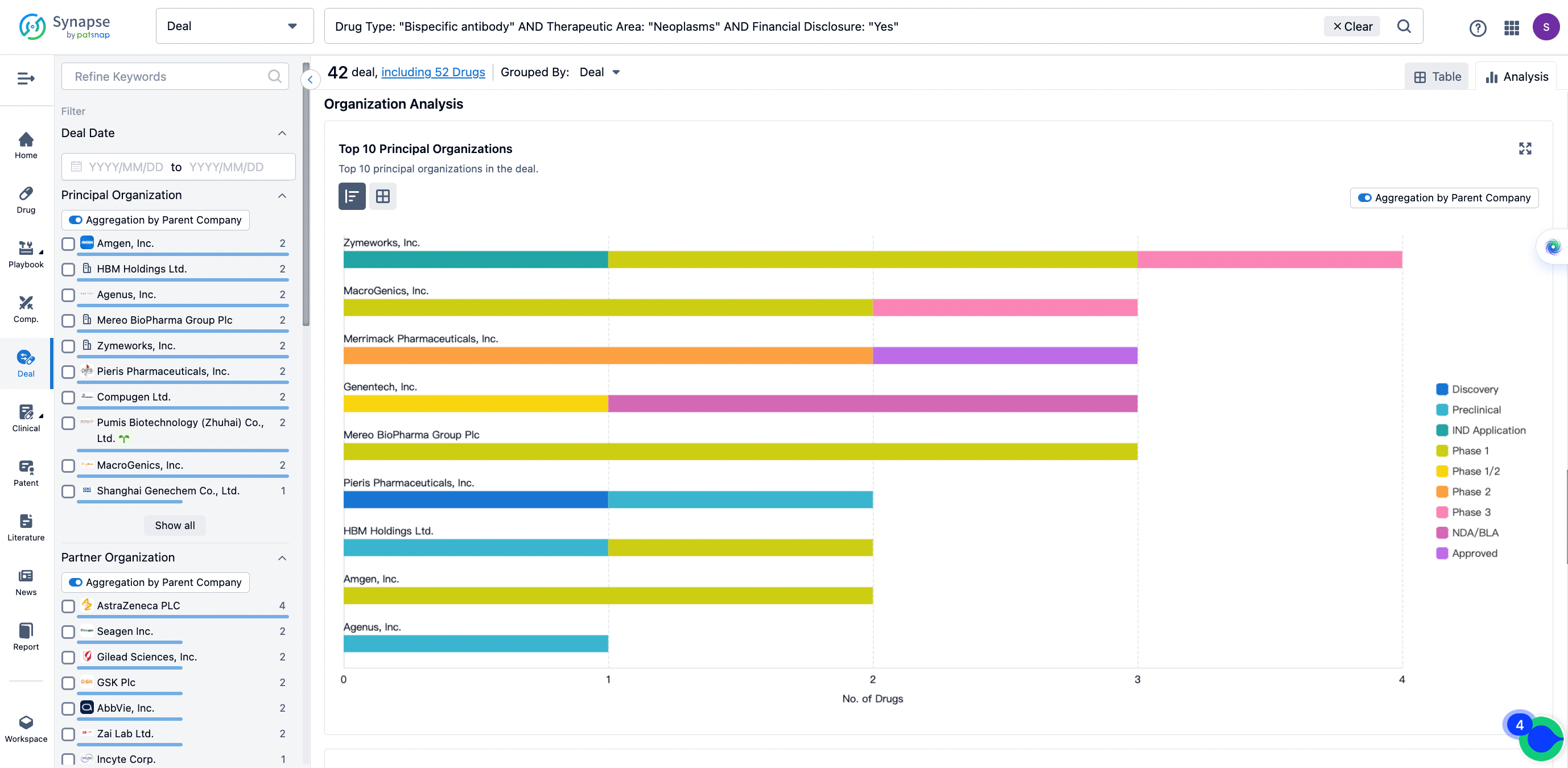
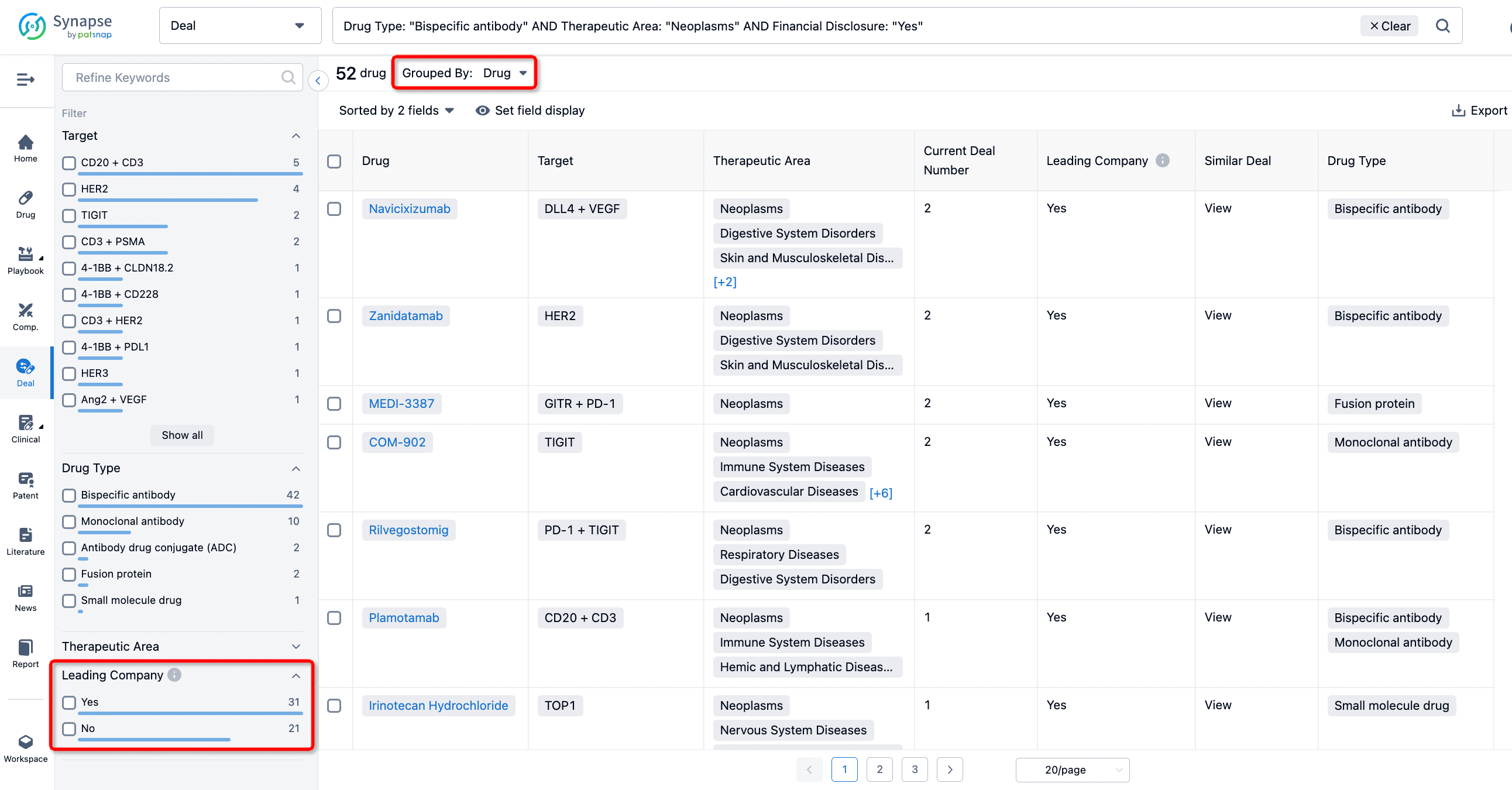
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
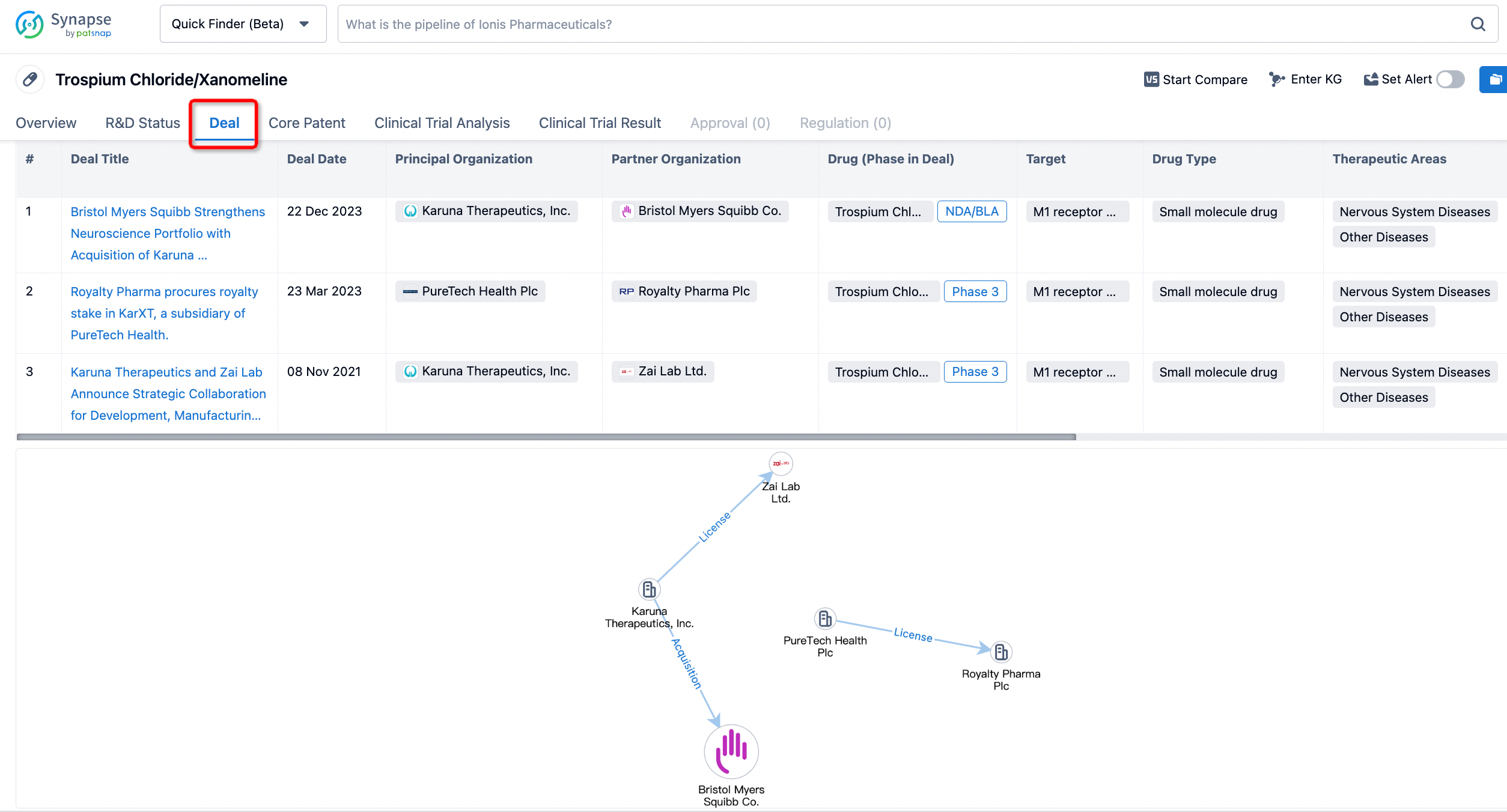
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to embark on a brand new journey of drug discovery!