EyePoint Pharma announces first patient treatment in early-phase VERONA trial for EYP-1901 in diabetic eye swelling
EyePoint Pharmaceuticals, Inc., an enterprise focused on advancing treatment options that aim to enhance the quality of life for individuals suffering from severe retinal conditions, has revealed the initiation of treatment for the first participant in the second-phase VERONA study. This trial is examining EYP-1901's efficacy for individuals with diabetic macular edema. The therapy EYP-1901, currently under examination, is a long-acting medicinal formulation that incorporates vorolanib, a specific inhibitor of tyrosine kinase, combined within a biodegradable Durasert E system.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Administering the initial dose to a participant in the Phase 2 VERONA study marks a pivotal step forward in our commitment to enhancing the quality of life for those suffering from severe retinal conditions. DME remains a prevalent visual impairment risk associated with diabetes and can culminate in profound vision deterioration. This disease is the second diabetic ocular condition under assessment in our research for its response to EYP-1901 as a potential therapy option," stated Jay Duker, M.D., at the helm of EyePoint Pharmaceuticals.
Dr. Duker added, "We are eager to share further progress regarding the EYP-1901 clinical endeavors, including the release of primary data from the Phase 2 PAVIA trial targeting non-proliferative diabetic retinopathy, which we expect by the second quarter of 2024, and the commencement of a groundbreaking Phase 3 trial focusing on wet age-related macular degeneration scheduled for the latter half of 2024."
The VERONA study is a Phase 2, randomized, controlled, single-masked clinical trial assessing EYP-1901 in patients with DME who have previously undergone the standard anti-VEGF therapy. The trial plans to include roughly 25 patients distributed across three groups: two different intravitreal dosages of EYP-1901 and an aflibercept comparator. The primary measurement for success in the VERONA study is the duration before a follow-up aflibercept injection is needed within a 24-week frame, based on pre-determined supplemental guidelines.
Additional measures in the trial encompass safety profiles, alterations in best corrected visual acuity, fluctuations in central subfield thickness as gauged by optical coherence tomography, and variations in the diabetic retinopathy severity score over time.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
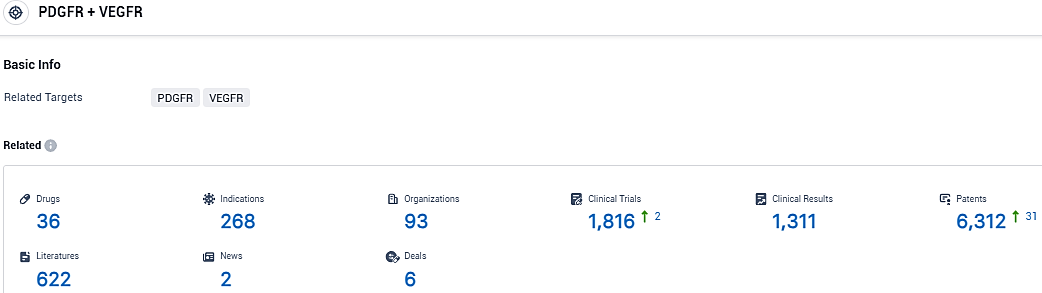
According to the data provided by the Synapse Database, As of January 16, 2024, there are 36 investigational drugs for the PDGFR and VEGFR target, including 268 indications, 93 R&D institutions involved, with related clinical trials reaching 1816, and as many as 6312 patents.
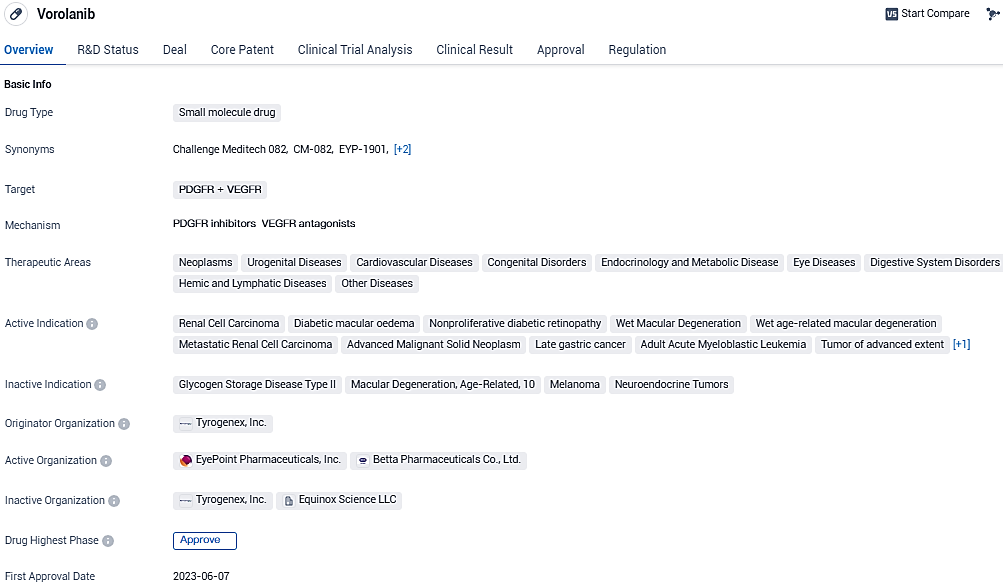
EYP-1901 is a small molecule drug that targets PDGFR and VEGFR, with a wide range of therapeutic applications in various disease areas. Its approval in China indicates its potential efficacy and safety profile.