FDA Approves Blincyto for CD19-Positive Philadelphia Chromosome-Negative B-ALL in Consolidation Stage
Amgen has revealed that the U.S. Food and Drug Administration has given approval for the use of BLINCYTO (blinatumomab) to treat both adult and pediatric patients aged one month or older who have CD19-positive Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia in the consolidation phase, independent of their measurable residual disease status.
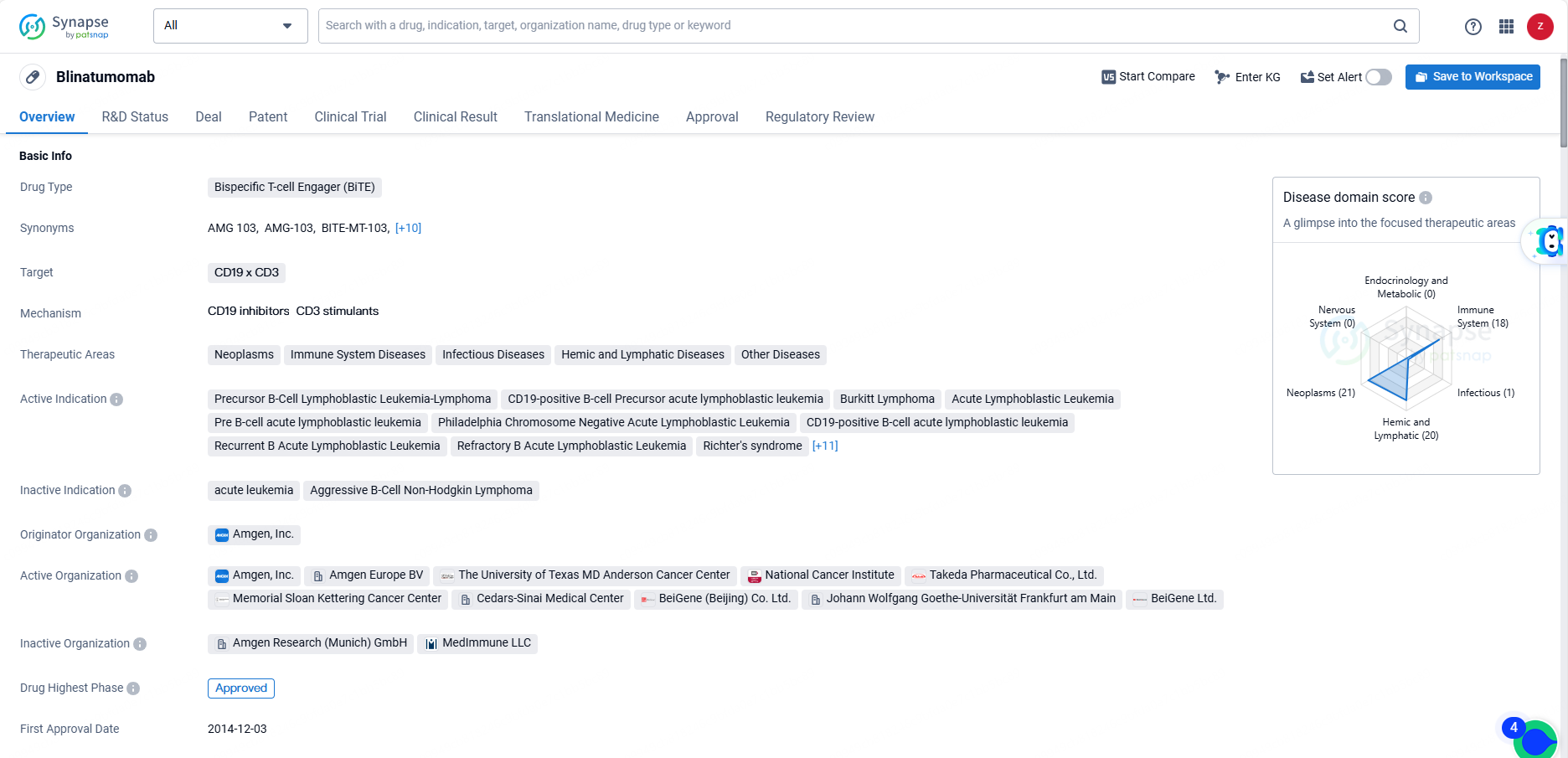
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
B-ALL is a severe form of blood cancer that has consistently high unmet medical needs. Over the past decade, BLINCYTO has benefited numerous B-ALL patients. "Today's approval for use in the frontline consolidation phase, irrespective of MRD status, enables us to assist a larger patient population with this groundbreaking, first-of-its-kind Bispecific T-cell Engager therapy," stated Jay Bradner, M.D., Executive Vice President, Research and Development, and Chief Scientific Officer at Amgen.
This approval represents the third indication for BLINCYTO and is largely based on data from the Phase 3 E1910 clinical trial led by ECOG-ACRIN Cancer Research Group. The study focused on newly diagnosed Philadelphia chromosome-negative B-ALL patients receiving post-induction consolidation therapy, aimed at deepening remission to secure sustained responses.
"In the E1910 trial, blinatumomab demonstrated a reduction in mortality risk and a significant enhancement in overall survival," remarked Selina M. Luger, M.D., Professor of Hematology-Oncology at the University of Pennsylvania’s Perelman School of Medicine and Abramson Cancer Center, Chair of the ECOG-ACRIN Leukemia Committee, and an investigator on the study. "This approval sets a new standard of care for B-ALL patients, giving them a more effective option compared to standard chemotherapy alone."
"The likelihood of B-ALL recurrence after initial treatment phases is fairly high, making this new approval quite significant for patients," commented E. Anders Kolb, M.D., President and CEO of The Leukemia & Lymphoma Society. "B-ALL is the most prevalent type of ALL, so having an effective option available earlier in the treatment process is crucial for clinicians aiming to give patients more time with their families."
The E1910 trial was designed and conducted independently of industry influence. Sponsored by ECOG-ACRIN using public funds from the National Cancer Institute (NCI), part of the National Institutes of Health, the study also included participation from other NCI-funded network groups. Additionally, Amgen supplied BLINCYTO and provided support via an NCI Cooperative Research and Development Agreement.
BLINCYTO is the first BiTE immuno-oncology therapy approved globally that targets CD19 antigens on B cells. BiTE molecules combat cancer by enabling the body’s immune system to recognize and target malignant cells, engaging T cells to attack cancer cells. By bringing T cells into proximity with cancer cells, T cells can release toxins and induce cancer cell death. BiTE immuno-oncology therapies are currently being evaluated for their potential to treat a broad range of cancers.
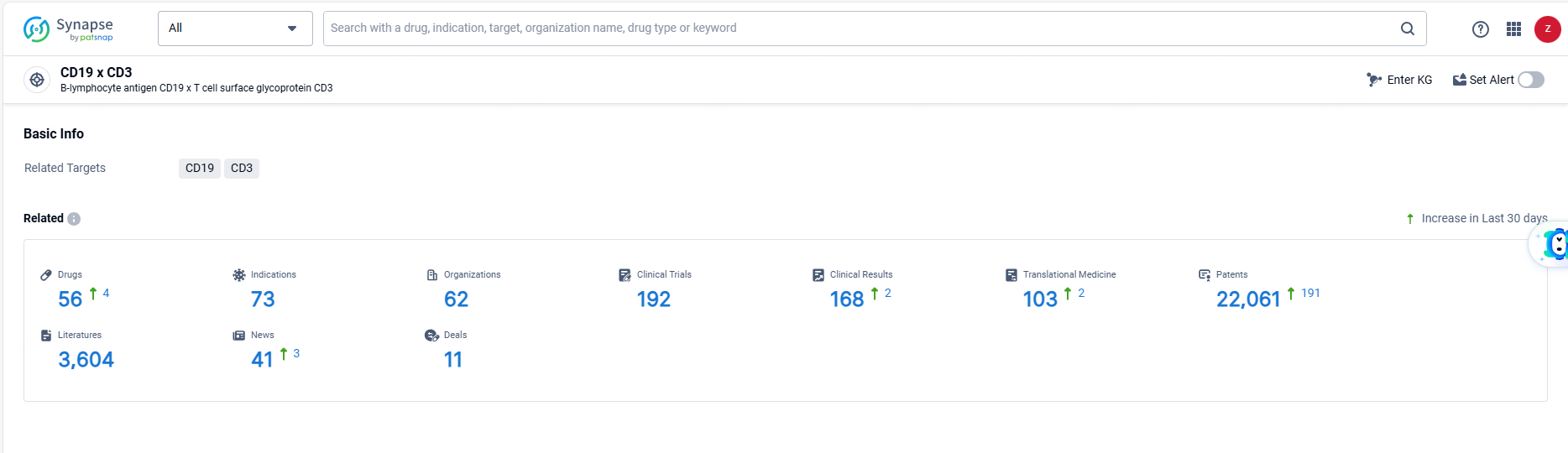
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 18, 2024, there are 56 investigational drugs for the CD19 and CD3 targets, including 73 indications, 62 R&D institutions involved, with related clinical trials reaching 192, and as many as 22061 patents.
Blinatumomab received its first approval in the United States in December 2014. The drug has been granted various regulatory designations, including Priority Review, Conditional marketing approval, Accelerated Approval, Orphan Drug, and Breakthrough Therapy. It has reached the highest phase of approval both globally and in China.