FDA Approves First Liquid Form of Recombinant Botulinum Toxin Type A
MingMed Biotechnology, a pioneering firm dedicated to the internal development and discovery of new pharmaceuticals, has recently announced that Claruvis Pharmaceutical Co., its partly owned subsidiary, has obtained approval from the U.S. Food and Drug Administration (FDA) for its Investigational New Drug (IND) application for YY003. This marks the approval for the world's first liquid recombinant BoNT/A formulation, packaged in a pre-filled syringe and designed for the treatment of glabellar lines.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are excited to have gained FDA clearance to progress the innovative liquid formulation of rBoNT/A (YY003) into clinical trials. This prefilled delivery system for the liquid-formulated recombinant BoNT/A offers a safer and more user-friendly alternative. By combining a super-pure, highly potent product with an easy-to-use design, BoNT/A users are provided with an optimized solution," stated Dr. Wu Yang, the Chief Scientific Officer at Claruvis. Dr. Yang brings over two decades of experience in botulinum toxin research and development to the company.
Claruvis Pharmaceutical, a leading biotech innovator in China, has achieved a global first with the development of a recombinant BoNT/A product advancing into clinical trials. The company recently concluded its Phase III clinical trial targeting moderate to severe glabellar lines, surpassing all study benchmarks. The trial's demonstrated efficacy, safety, and immunogenicity profiles mark a significant breakthrough in the cosmetic application of BoNTs. On September 14th, Claruvis initiated dosing for the first participant in a Phase II trial addressing adult upper arm spasms in China, setting another mondial record for the therapeutic use of recombinant BoNT/A. The firm has embarked on its inaugural Biologics License Application (BLA) in China, supported by a suite of successful Phase I, II, and III clinical results.
Dr. Yang further commented, "The FDA's approval for clinical trials of YY003 is a crucial step towards introducing a top-tier product in the Botulinum toxin sector."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
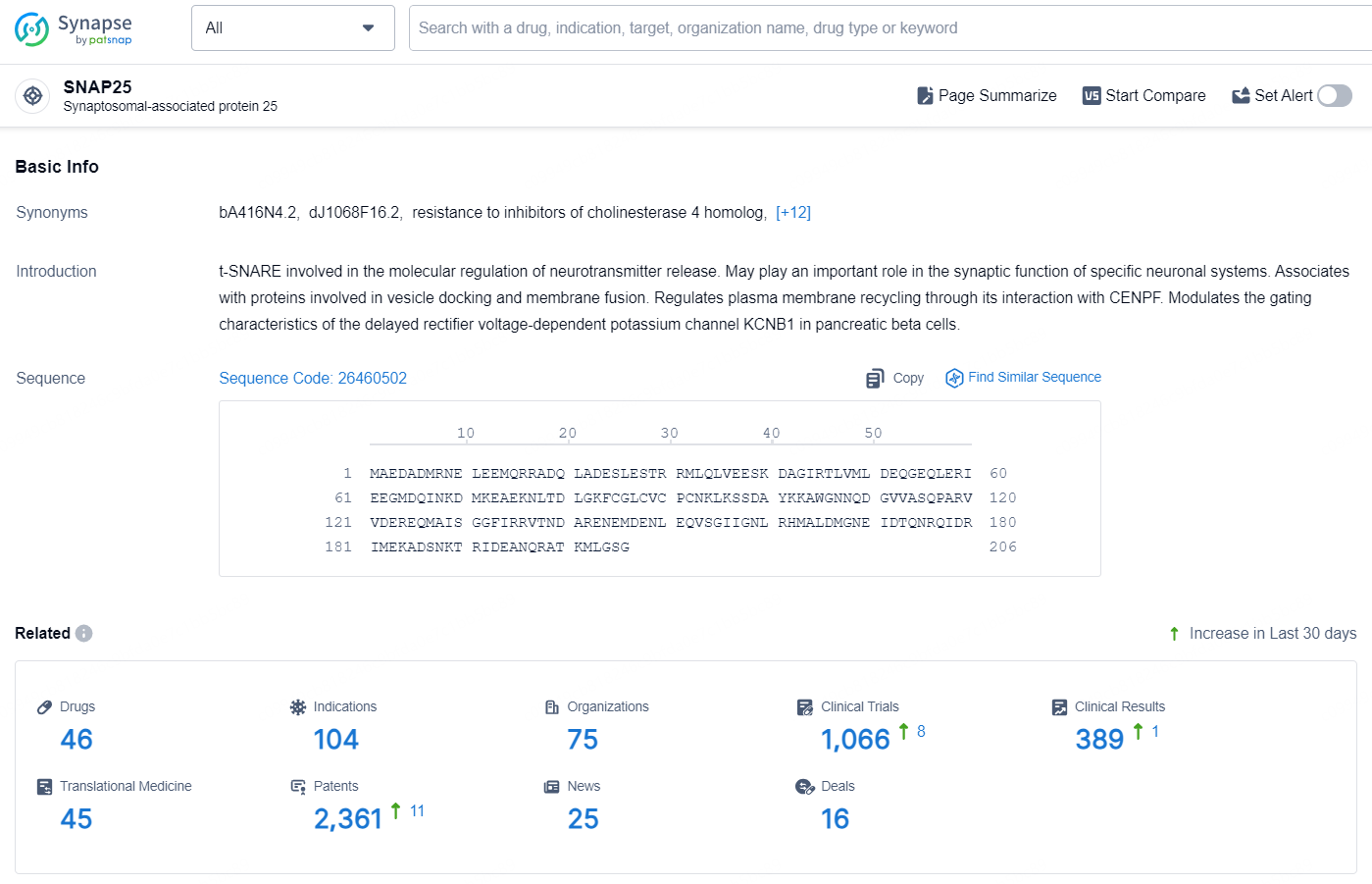
According to the data provided by the Synapse Database, As of September 29, 2024, there are 46 investigational drug for the SNAP25 targets, including 104 indications, 75 R&D institution involved, with related clinical trials reaching 1066, and as many as 2361 patents.
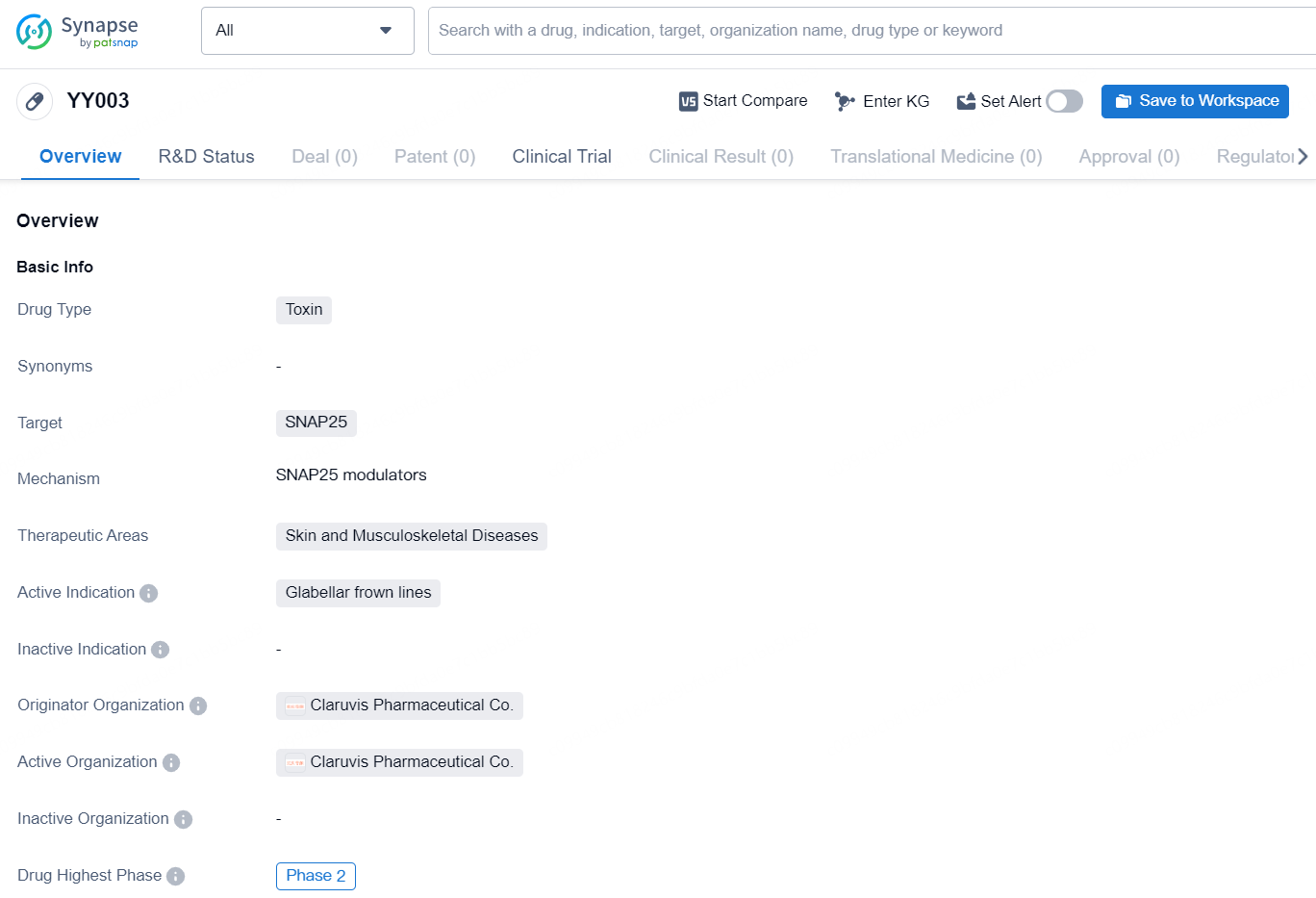
YY003 is a toxin drug developed by Claruvis Pharmaceutical Co., targeting the SNAP25 protein. The drug is specifically designed to treat Glabellar frown lines, which are wrinkles located between the eyebrows. This drug falls under the therapeutic areas of Skin and Musculoskeletal Diseases. In terms of its development, YY003 has reached the highest global phase of Phase 2, indicating that it has undergone significant preclinical and early clinical testing.