FDA Approves Galapagos' IND for Phase 1/2 Trial of GLPG5101, a CD19 CAR-T Therapy
Galapagos NV (Euronext & NASDAQ: GLPG) reported that the U.S. Food and Drug Administration (FDA) has approved their Investigational New Drug (IND) application for ATALANTA-1. This Phase 1/2 multicenter trial is designed to assess the feasibility, safety, and effectiveness of GLPG5101 in patients who have relapsed or are refractory to treatment for non-Hodgkin lymphoma (R/R NHL).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
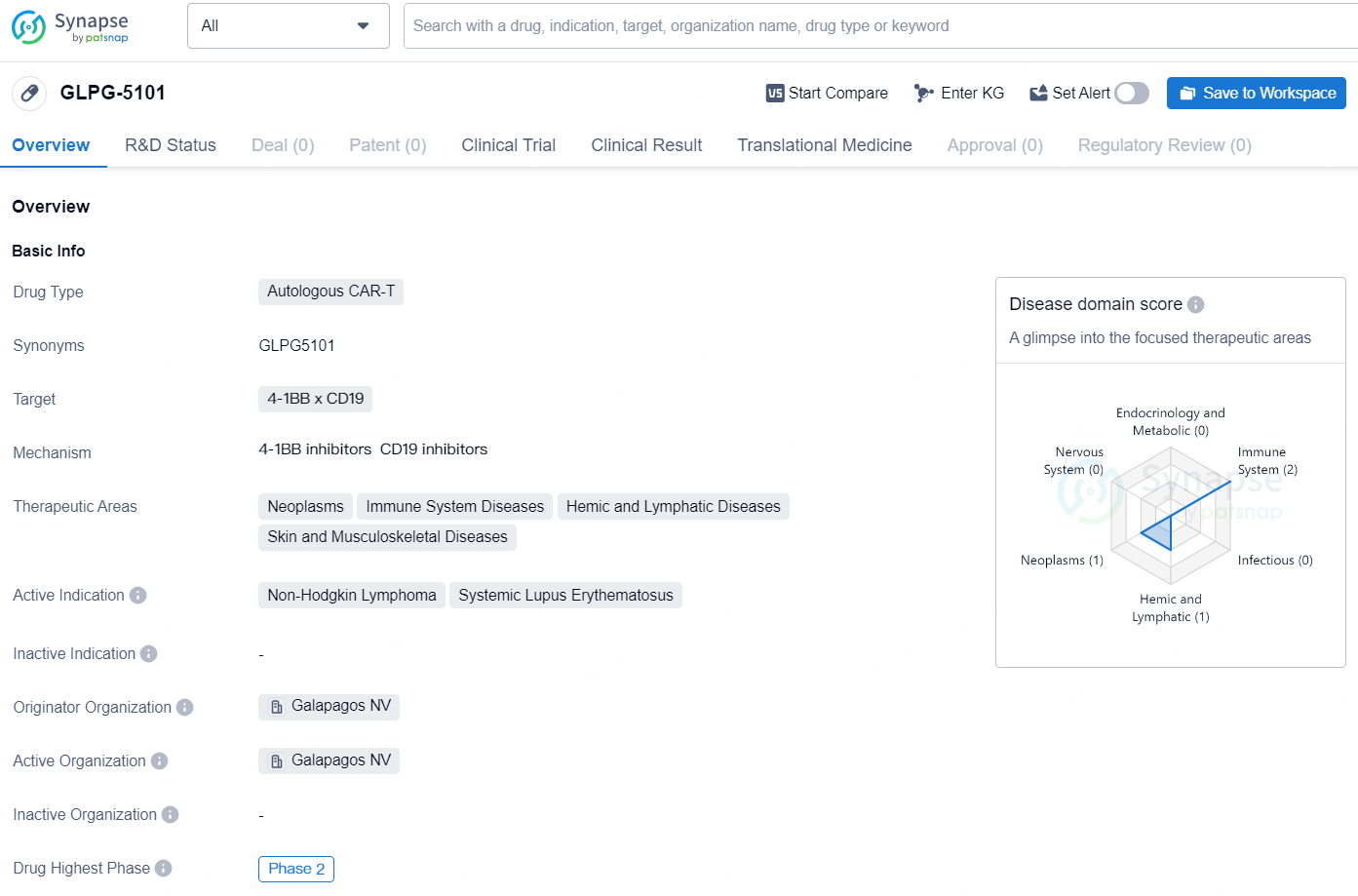
GLPG5101, an autologous CD19 CAR-T cell therapy candidate, is developed using Galapagos’ cutting-edge decentralized cell therapy production platform. This approach allows for the potential administration of fresh, viable cells within a median vein-to-vein timeframe of seven days.
The main goal of the Phase 1 segment of the ATALANTA-1 trial is to assess the safety and initial efficacy of GLPG5101 to establish the optimal dose for Phase 2. Additional aims include evaluating the efficacy and practicality of the decentralized production of GLPG5101. For Phase 2, the primary objective is to determine the objective response rate, while secondary objectives encompass the complete response rate, response duration, progression-free survival, overall survival, safety, pharmacokinetics, and the feasibility of decentralized manufacturing. Patients will be monitored for a duration of 24 months.
The ongoing Phase 1/2 ATALANTA-1 study in Europe has shown promising early outcomes in individuals with R/R NHL.
"We are committed to propelling innovative advancements that expand the accessibility of cell therapies for patients facing rapidly advancing cancers," stated Dr. Paul Stoffels, CEO and Chairman of the Board of Directors at Galapagos. "Our pioneering decentralized manufacturing platform is crafted to address numerous obstacles encountered by current CAR-T production technologies. This platform from Galapagos promises enhanced speed and scalability, providing fresh, viable cells within a seven-day median vein-to-vein period, directly to patients. The IND clearance for the Phase 1/2 GLPG5101 study represents a crucial advancement in our cell therapy program, progressing us towards delivering our CD19 CAR-T cell therapy to U.S. patients."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
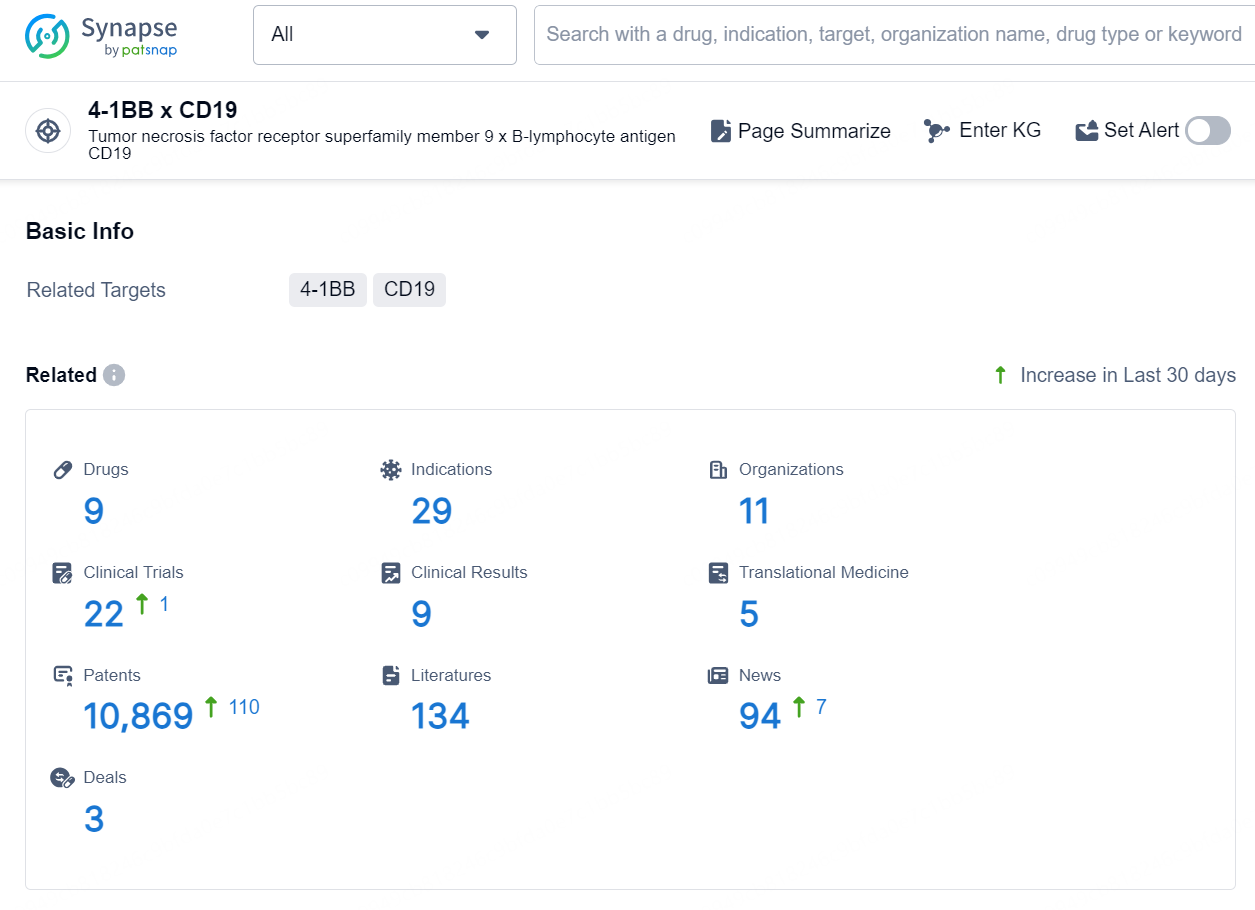
According to the data provided by the Synapse Database, As of August 27, 2024, there are 9 investigational drugs for the 4-1BB x CD19 targets, including 29 indications, 11 R&D institutions involved, with related clinical trials reaching 22, and as many as 10869 patents.
GLPG-5101 is an autologous CAR-T drug that targets 4-1BB x CD19 and is being developed by Galapagos NV. The therapeutic areas that this drug aims to treat include neoplasms, immune system diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. The active indications for GLPG-5101 are Non-Hodgkin Lymphoma and Systemic Lupus Erythematosus. This drug is currently in the Phase 2 stage of development, which represents a significant advancement in its clinical testing. Phase 2 trials involve a larger group of patients and are conducted to further evaluate the drug's safety and efficacy.