FDA approves PADCEV® and KEYTRUDA® combo treatment for advanced bladder cancer
Pfizer Inc. along with Astellas Pharma Inc. disclosed on the 15th of December, 2023, that PADCEV® (enfortumab vedotin-ejfv), an innovative antibody-drug conjugate (ADC), in conjunction with KEYTRUDA® (pembrolizumab), a known PD-1 blocking agent, has obtained authorization from the U.S. FDA. This approval permits the use of these pharmaceuticals in managing urothelial carcinoma that has either spread to nearby tissues or distant areas (la/mUC) in adult individuals.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This novel therapy represents a groundbreaking option beyond traditional platinum-based chemotherapy regimens, which have long been the accepted primary treatment for initial cases of advanced urothelial carcinoma (la/mUC). This new endorsement rests on pivotal data from the EV-302 Phase 3 clinical trial, also referred to as KEYNOTE-A39. The study revealed that this new therapeutic duo significantly extended both the median duration of overall survival and the median time to disease progression in patients with la/mUC who had not received prior treatment, when measured against the outcomes of platinum-based chemotherapy.
The European Society for Medical Oncology Congress 2023 was the venue where the EV-302 trial outcomes were showcased. Further cementing its importance, EV-302 fulfills the criteria for confirmatory trials for the accelerated authorization granted in the United States for this drug combination in the treatment of adult la/mUC patients who are ineligible for cisplatin-based chemotherapy—this authorization was granted in April 2023. Additionally, the approval broadens the approved use to those patients who may be candidates for cisplatin chemotherapy. Moreover, these trial results are being utilized as a foundation for regulatory submissions worldwide.
Speaking on the impact of the Phase 3 EV-302 study, Dr. Roger Dansey, the Chief Development Officer of Oncology at Pfizer, commented, "The pairing of PADCEV and pembrolizumab has shown a pronounced improvement in patient survival outcomes in advanced bladder cancer, offering nearly twice the median OS and PFS than that observed with traditional chemotherapy. Our aspiration is that this newly approved therapy will redefine the treatment benchmarks for advanced bladder cancer and provide patients additional quality time with their families."
Dr. Ahsan Arozullah, who is the Senior Vice President and leads Oncology Development at Astellas, remarked on the significance of this FDA approval. He highlighted that it marks a fundamental shift in how advanced bladder cancer is managed and offers a beacon of hope to the many U.S. residents battling this challenging condition. "It is a remarkable advancement," he noted, "being the first treatment approach in the realm of advanced urothelial cancer to demonstrate effectiveness that surpasses the longstanding, platinum-based standard of care."
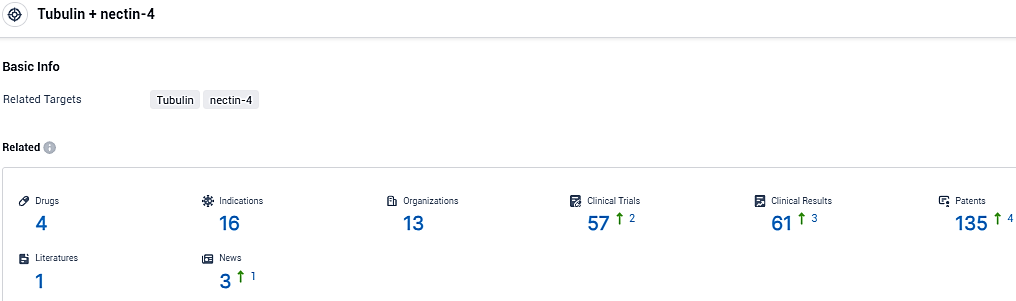
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 27, 2023, there are 4 investigational drugs for the Tubulin and nectin-4 target, including 16 indications, 13 R&D institutions involved, with related clinical trials reaching 57, and as many as 135 patents.
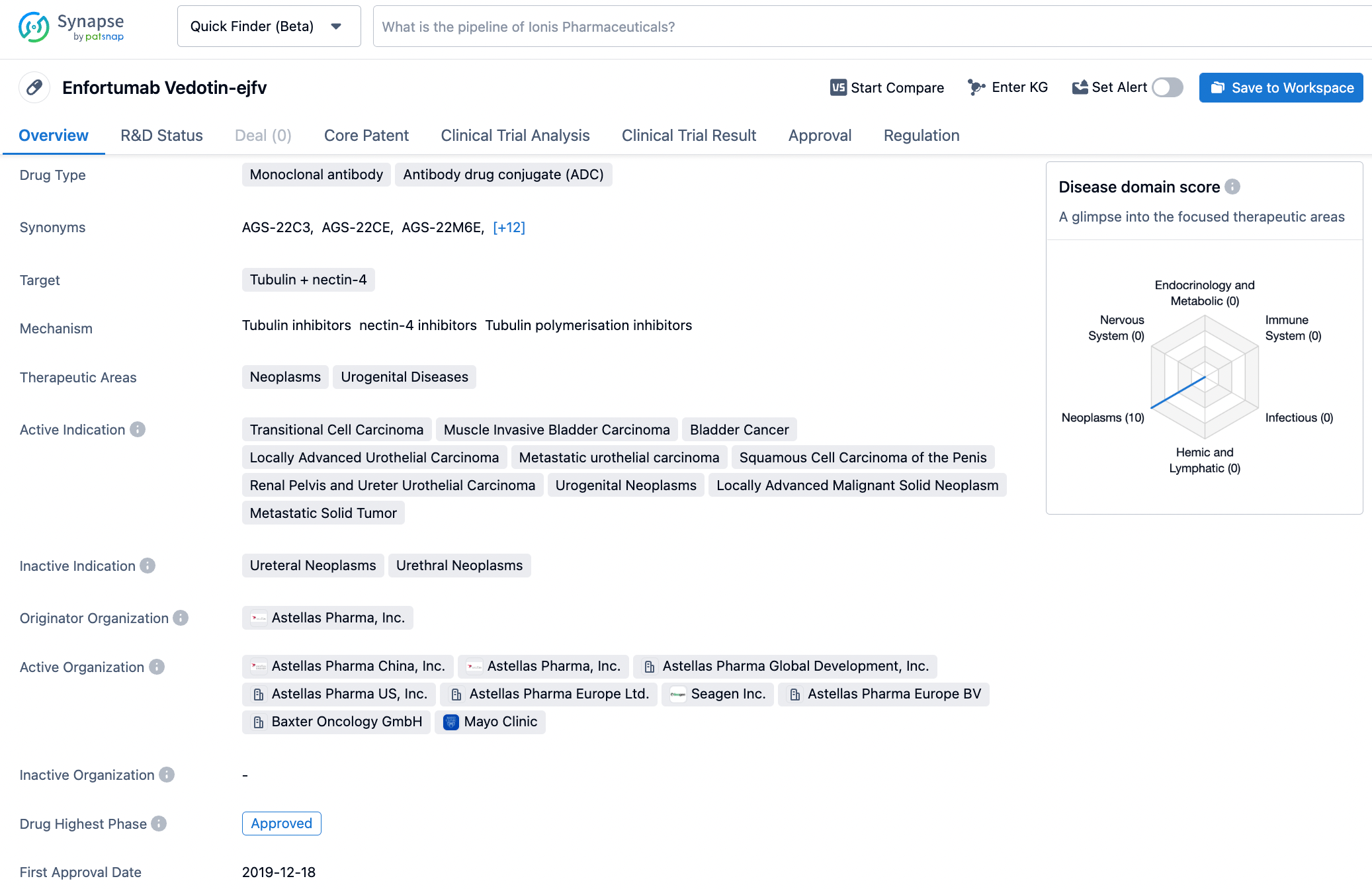
PADCEV (enfortumab vedotin-ejfv) is a first-in-class antibody-drug conjugate that is directed to Nectin-4, a protein located on the surface of cells and highly expressed in bladder cancer.vi Nonclinical data suggest the anticancer activity of PADCEV is due to its binding to Nectin-4-expressing cells, followed by the internalization and release of the anti-tumor agent monomethyl auristatin E into the cell, which result in the cell not reproducing and in programmed cell death.