FDA Approves Servier's VORANIGO®, First Targeted Treatment for Grade 2 IDH-mutant Glioma
Servier revealed that the U.S. Food and Drug Administration has given the green light to VORANIGO®, an inhibitor of isocitrate dehydrogenase-1 ((IDH1) and isocitrate dehydrogenase-2 (IDH2). This drug is intended for use in treating both adult and pediatric patients aged 12 and above who have Grade 2 astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation, after undergoing surgery such as biopsy, sub-total resection, or gross total resection.
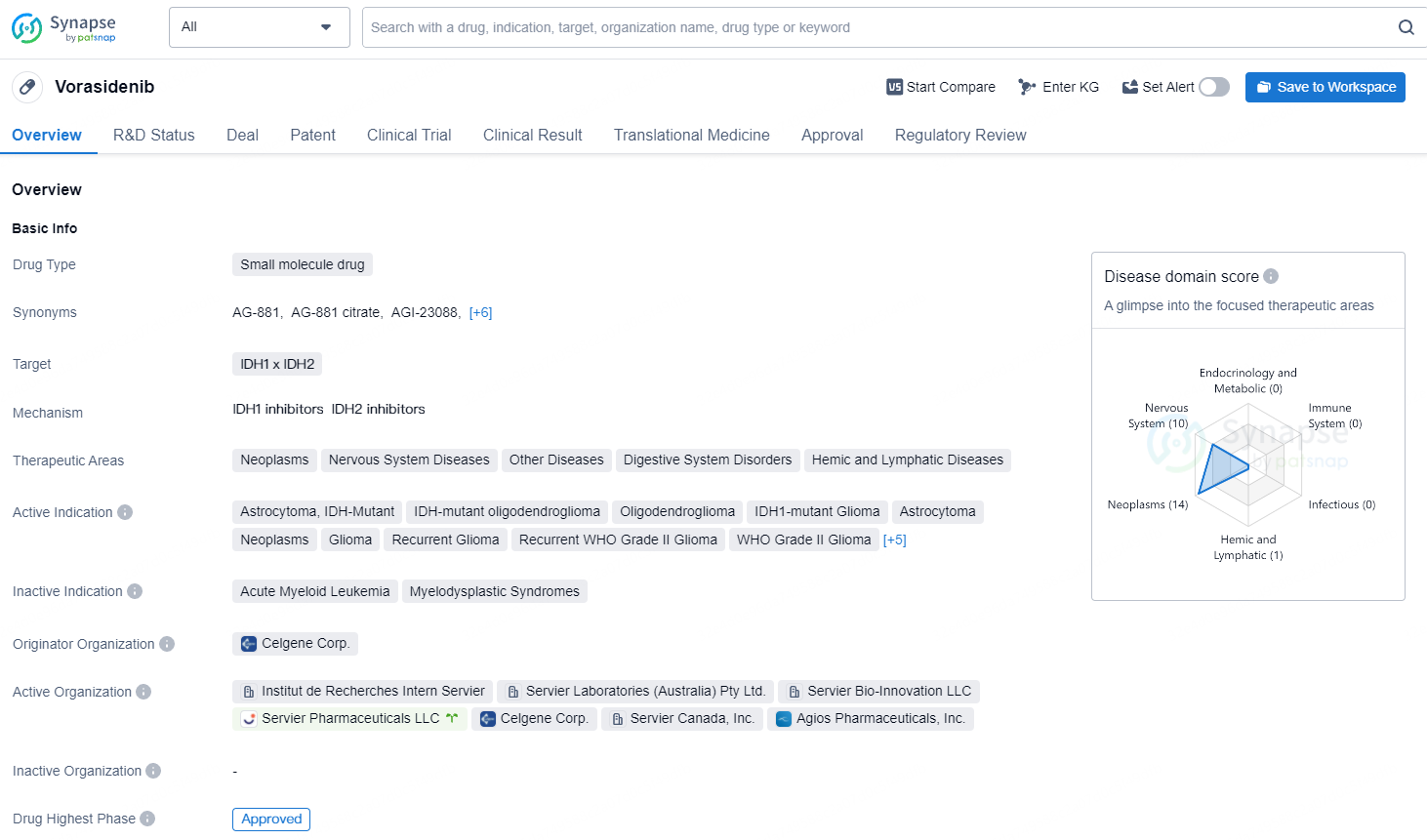
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Gliomas are forms of brain cancer that impede normal brain activities and bring about a range of symptoms. Diffuse gliomas with IDH mutations are recognized as the most prevalent malignant primary brain tumors diagnosed in adults under the age of 50. These tumors are not curable with existing treatments and, without intervention, they continue to grow and invade healthy brain tissues.
"The approval of VORANIGO represents a significant advance in cancer treatment and is a landmark event for individuals with Grade 2 IDH-mutant glioma," stated Arjun H. Prasad, Chief Commercial Officer at Servier Pharmaceuticals.
"VORANIGO, the first major development in this particular disease in almost 25 years, provides patients with an unparalleled extension in progression-free survival. We are honored to bring this unique therapy to those in need and remain dedicated to introducing innovative targeted treatments for cancer patients," added Arjun H. Prasad.
In normal human cells, a set of genes known as isocitrate dehydrogenases assist in the breakdown of nutrients and energy production for cells. Mutations in IDH1 and IDH2 are linked to various cancers, preventing cells from differentiating into their destined specialized types.
"Individuals with Grade 2 IDH-mutant gliomas have long confronted the difficult reality of an incurable condition with very limited treatment options following surgery," remarked Ralph DeVitto, President & CEO of the American Brain Tumor Association. "The FDA approval of VORANIGO is a momentous advancement in glioma therapy, providing renewed optimism for patients and their families affected by this persistent disease."
The approval of VORANIGO is underpinned by findings from the critical Phase 3 INDIGO clinical trial documented in *The New England Journal of Medicine* and presented during the Plenary Session at the 2023 Annual Meeting of the American Society of Clinical Oncology, which demonstrated that VORANIGO considerably prolonged progression-free survival and the interval to the next intervention compared to a placebo.
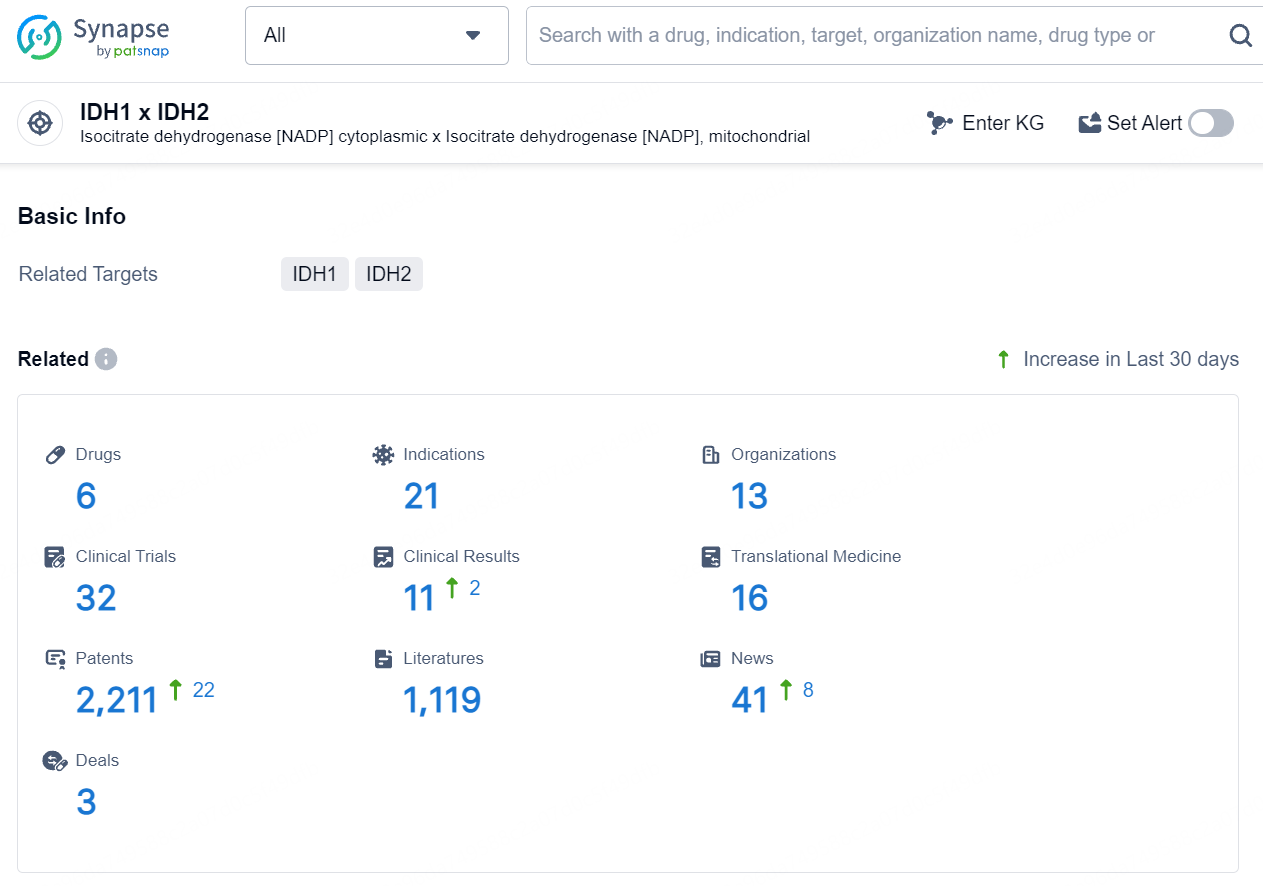
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 12, 2024, there are 6 investigational drugs for the IDH1 and IDH2 target, including 21 indications, 13 R&D institutions involved, with related clinical trials reaching 32, and as many as 2211 patents.
Vorasidenib represents a promising development in the pharmaceutical industry, with its potential to address a wide range of neoplastic and nervous system diseases, as well as other related disorders. Its advanced regulatory status and approval in the United States demonstrate its potential to positively impact patient care and treatment outcomes in the near future.