FDA approves Transcenta Phase III Study of Osemitamab for Gastric/Gastroesophageal Cancer
Transcenta Holding Limited, a firm conducting clinical stage biopharmaceutical operations that caters to the discovery, research, development, and production of antibody-mediated treatments, states that it has received the green light from FDA to continue with TranStar 301, which is a global pivotal Phase III trial.
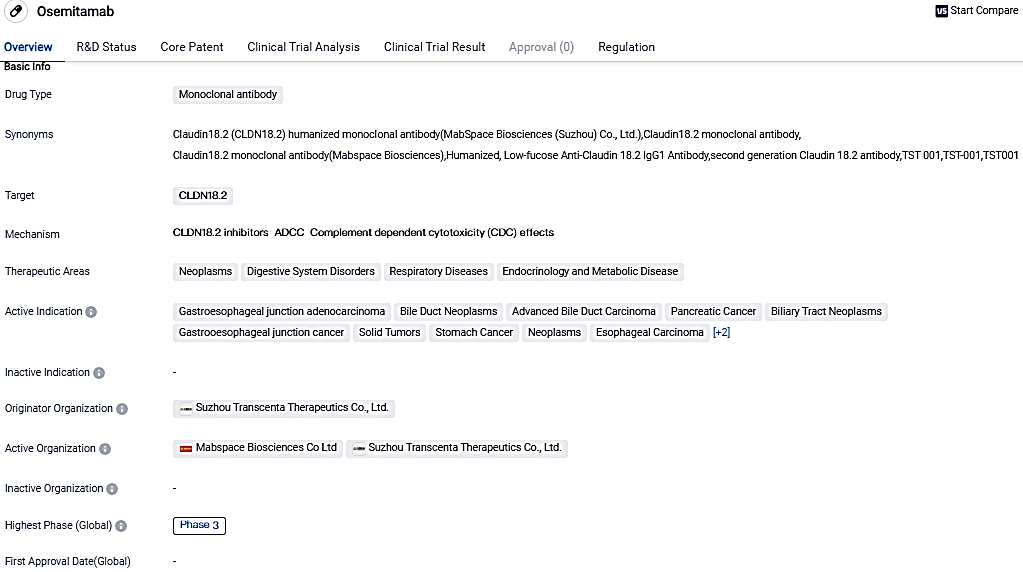
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This trial is conducted for Osemitamab (TST001) used together with Nivolumab and chemotherapy. This is employed as the primary treatment for patients suffering from advanced or metastatic gastric or gastroesophageal adenocarcinoma, which expresses HER2-negative and CLDN18.2.
This pivotal point signifies a significant leap in Osemitamab's worldwide progression, marking a novel milestone, in addition to the authorizations given by CDE in China and MFDS in South Korea as of July 2023, for its Phase III principal trial.
This step in Osemitamab's path indicates a promising future making it an international therapeutic solution, elevating the current universal medical protocol for HER2-negative metastatic gastric or gastroesophageal adenocarcinoma treatment. By strategically aiming at CLDN18.2 and amalgamating it with Nivolumab and chemotherapy, Osemitamab is ready to redefine the G/GEJ cancer treatment approach.
Gastric cancer remains a significant global cancer type, causing more than a million new cases in 2020, and an expected 769,000 deaths, placing it at the fifth highest incidence rate and fourth highest mortality rate worldwide. The primary structure of the chemotherapy program for patients having HER2-negative advanced gastric cancer comprises combinations of platinum and fluoropyrimidine.
Nivolumab received approval for its combination with chemotherapy as a treatment strategy for patients with advanced or metastatic gastric cancer. Despite the enhanced treatment results, nivolumab and chemotherapy's median overall survival rate remains less than 14 months.
In an effort to support their global Phase III trial application and the FDA EOP2 meeting, Transcenta has launched Phase II clinical trials in the U.S and China, involving the combination of Osemitamab with chemotherapy or nivolumab. Along with this, they have experimented using numerous Osemitamab doses to determine the most effective dosage for the global Phase III trial. Additionally, Transcenta has worked with a renowned CDx developer in the U.S to develop a CLDN18.2 specific companion diagnostic assay.
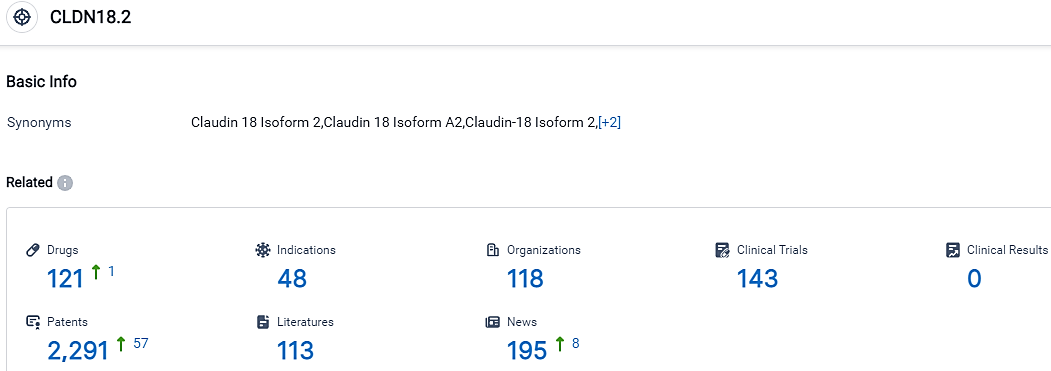
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 14, 2023, there are 121 investigational drugs for the CLDN18.2 target, including 48 indications, 118 R&D institutions involved, with related clinical trials reaching 143,and as many as 2291 patents.
Osemitamab is a second-generation humanized CLDN18.2 targeting antibody with improved CLDN18.2 binding affinity and enhanced antibody-dependent cellular cytotoxicity. It has shown anti-tumor activities in preclinical tumor models with a broad range of CLDN18.2 expression.