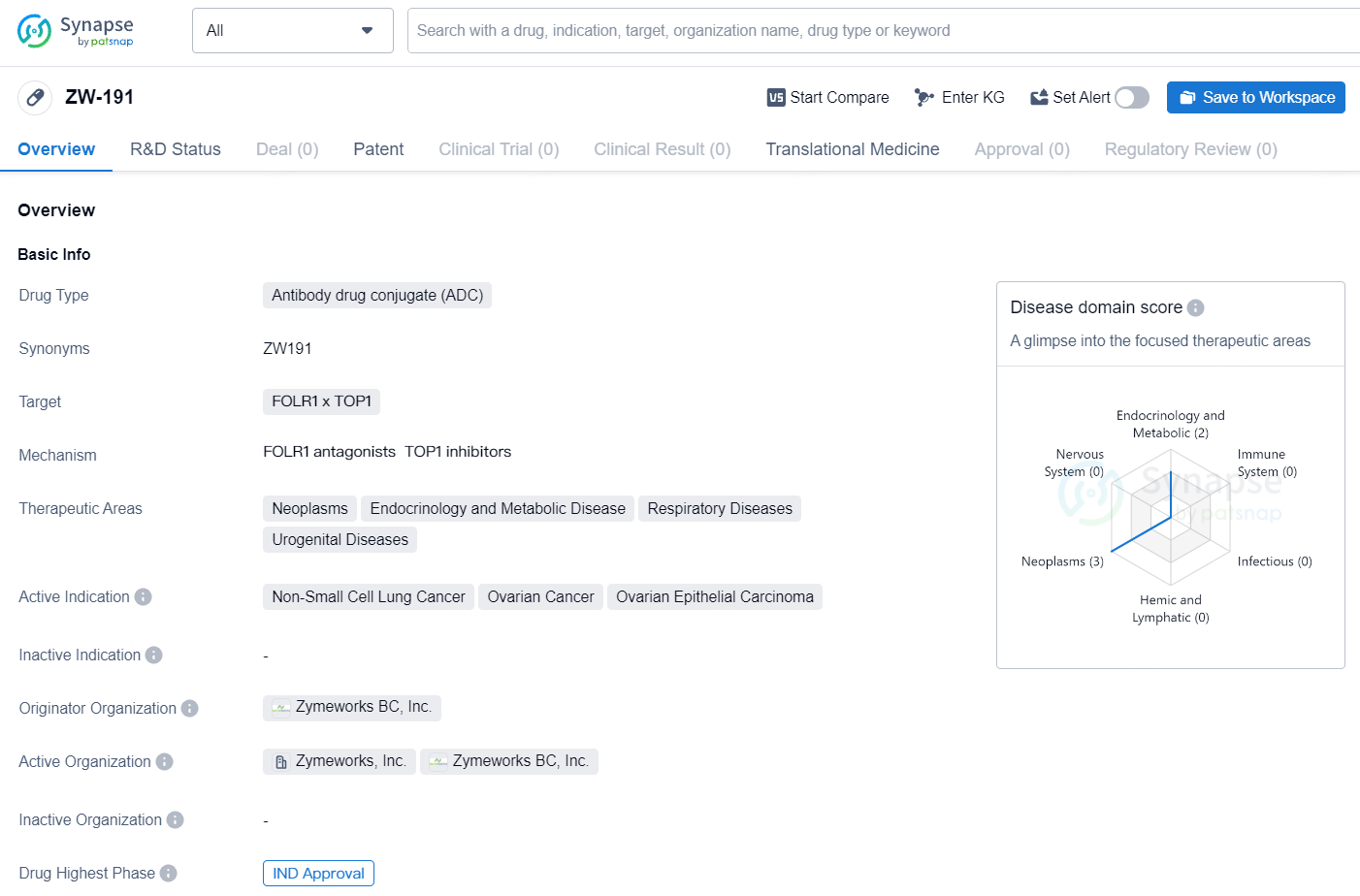
FDA Approves Zymeworks' ZW191, a Novel Folate Receptor-⍺ Targeting Topoisomerase I Inhibitor ADC
Zymeworks Inc., a biotechnology firm in the clinical stage working on an array of innovative, multifunctional biotherapeutics aimed at enhancing treatment standards for challenging diseases, has announced that the United States Food and Drug Administration has approved the investigational new drug application for ZW191. This innovative folate receptor-⍺ (FR⍺) targeted topoisomerase I inhibitor (TOPO1i) antibody-drug conjugate is a novel product by the Company.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Paul Moore, Chief Scientific Officer of Zymeworks, remarked, "ZW191 stands out in our lineup as a distinct product candidate, underscoring the efficacy of our strategy to develop leading antibody-drug conjugates. Crafted to target FR⍺, present in various challenging-to-treat cancers, ZW191 features an innovative antibody and drug-linker combination. This results in a unique blend of antibody-linker stability and payload potency, alongside significant bystander activity, potentially improving efficacy and enabling the targeting of lower FR⍺ levels than earlier drug candidates. We're excited to hit this R&D milestone shortly after our recent FDA clearance for ZW171 in June and anticipate embarking on clinical development for both ZW191 and ZW171 in 2024."
ZW191 was engineered using the Company's drug conjugate platforms, notably the novel TOPO1i-based payload technology, ZD06519, aimed at FR⍺-expressing tumors, such as ovarian and other gynecological cancers, as well as non-small cell lung cancer. An optimal drug-antibody ratio of eight was selected to balance efficacy and tolerability.
The FR⍺ monoclonal antibody integrated in ZW191 was internally developed and chosen for its superior internalization properties, allowing it to target high, mid, and low levels of FR⍺ expression. FR⍺ is a clinically validated target present in about 75% of ovarian carcinomas and 70% of NSCLC. ZW191 has shown strong anti-tumor activity and maintained a favorable safety profile in preclinical trials.
The Company anticipates filing applications for regulatory approval to start clinical trials for ZW191 outside the US in the latter half of 2024. ZW191 is the first of three ADC molecules incorporating the Company's unique ZD06519 payload set for clinical trials, with IND applications for ZW220 and ZW251 planned for 2025.
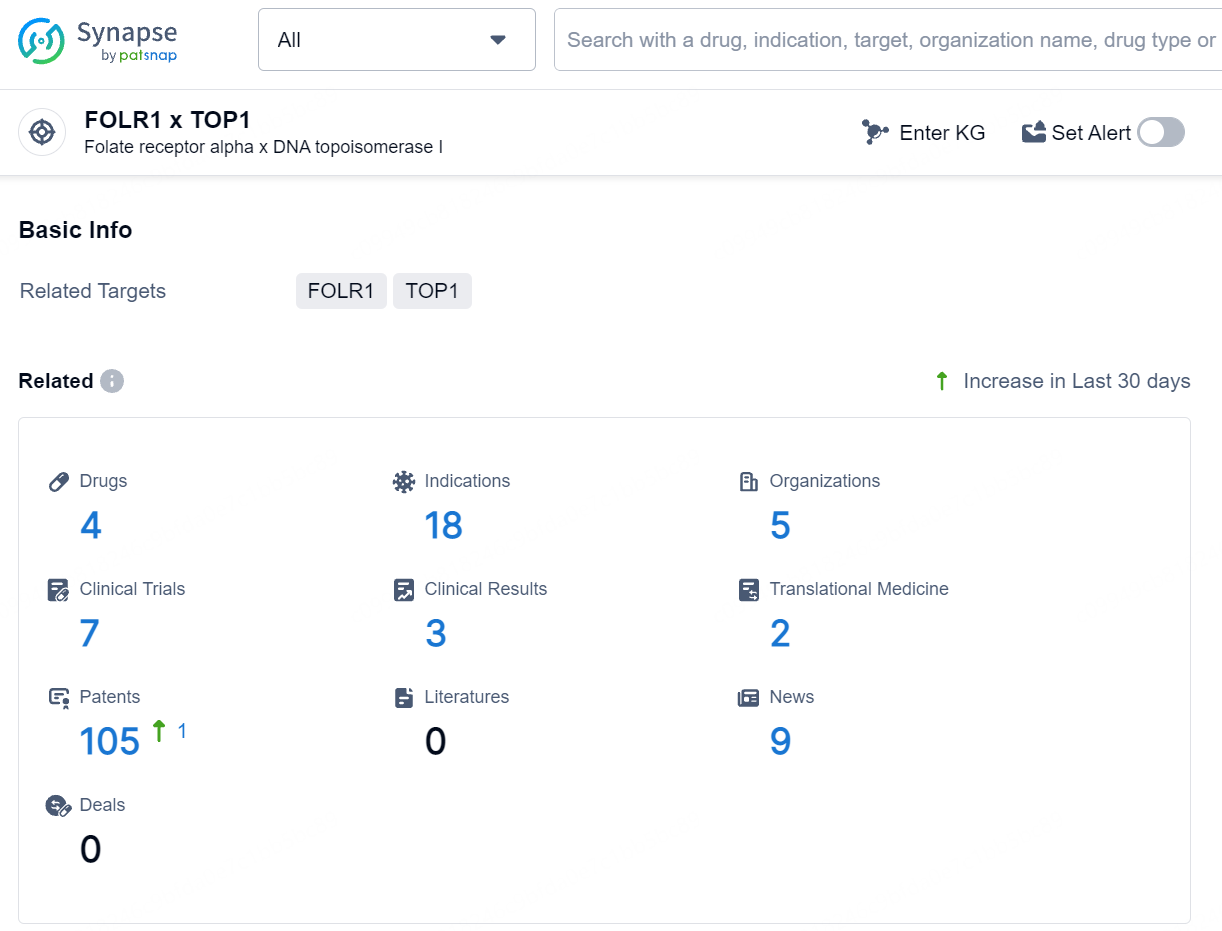
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 25, 2024, there are 4 investigational drugs for the FOLR1 and TOP1 target, including 18 indications, 5 R&D institutions involved, with related clinical trials reaching 7, and as many as 105 patents.
ZW-191 represents an innovative approach to cancer therapy through the use of antibody drug conjugates, and its progression to the IND approval stage indicates a promising development in the pharmaceutical industry, with potential implications for the treatment of non-small cell lung cancer, ovarian cancer, and ovarian epithelial carcinoma.