FDA Authorizes ARTHEx for Early ATX-01 Trial in Myotonic Dystrophy under ArthemiR™
Spanning the realm of clinical-stage biotechnology with a keen eye on pioneering therapeutic solutions via microRNA modulation, ARTHEx Biotech S.L. has disclosed the recent endorsement from the U.S. Food and Drug Administration. This official green light grants the enterprise the authorization to embark on the foremost Phase I-IIa clinical trial dubbed ArthemiR™, which will evaluate the potential of their drug candidate ATX-01 in addressing the complexities of Myotonic Dystrophy Type 1.
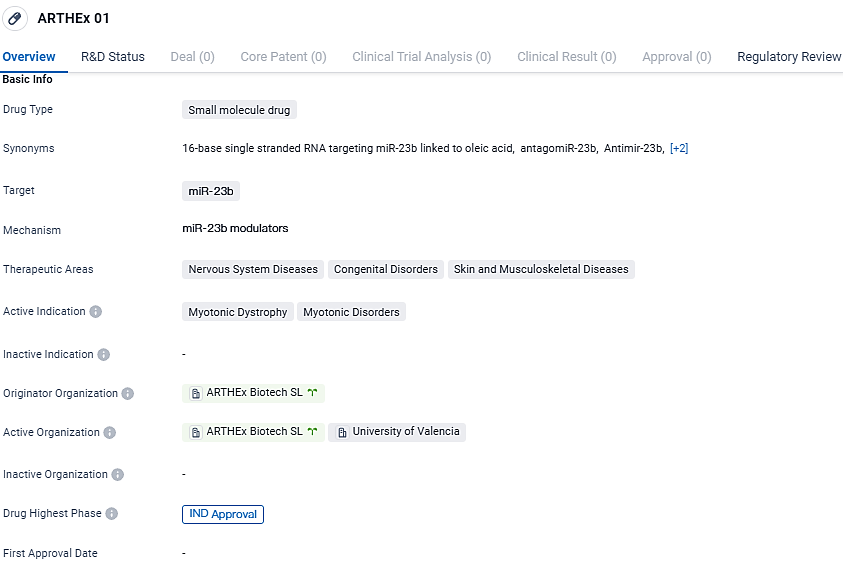
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ATX-01, a specifically engineered antimiR aimed at microRNA 23b (miR-23b), marks the premiere of a microRNA-focused treatment being assessed for Myotonic Dystrophy Type 1 (DM1) and is also the inaugural therapy from ARTHEx to advance to clinical stages. miR-23b has been identified to play a role in controlling MBNL protein levels that contribute to the development of DM1, an infrequent but severe neuromuscular condition characterized by failing muscle strength and debilitating symptoms. Presently, there is an absence of any therapeutic interventions that can alter the course of DM1.
Chief Medical Officer Dr. Judith Walker of Arthex shared, "The authorization from the FDA to commence our very first human trials, under the name ArthemiR™ for the application of ATX-01 in battling DM1, signifies a crucial progression for both ARTHEx and for those affected by DM1, including patients and their relatives who are awaiting an effective treatment option."
The ArthemiR™ investigation is designed as a Phase I-IIa trial, incorporating both blinded and placebo-controlled methodologies with an escalating dose approach. It aims to recruit individuals diagnosed with the classic form of Myotonic Dystrophy Type 1. The pivotal goal is to appraise the safety profile and determine how well different doses of ATX-01 can be endured by individuals with DM1. The research spearheaded by ARTHEx will further look into the engagement of the therapeutic target within the muscle tissue by assessing indicators such as MBNL concentration and splicing patterns.
Professor and Vice Chair of Research at Virginia Commonwealth University's Department of Neurology, Dr. Nicholas Johnson, who is also at the helm of the ArthemiR™ study, remarked, "The ushering in of innovative agents into clinical evaluations is a matter of great enthusiasm, primarily those that exhibit diverse action modalities. There is a strong desire among patients for novel clinical studies, and we are thrilled at the prospect of commencing participant inclusion shortly."
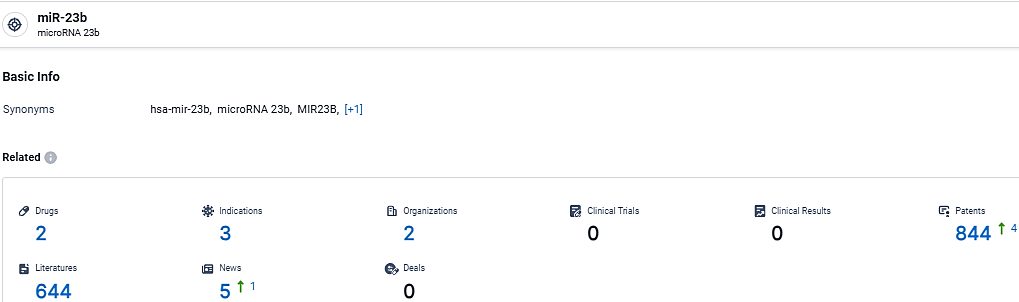
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 4, 2024, there are 2 investigational drugs for the miR-23b target, including 3 indications, 2 R&D institutions involved, and as many as 844 patents.
ATX-01 is an antimiR oligonucleotide designed to target microRNA 23b, which is associated with regulating the expression of MBNL proteins involved in the pathogenesis of DM1. ATX-01 will shortly be evaluated in the Phase I-IIa ArthemiR™ trial for the treatment of DM1. ATX-01 has received Orphan Drug Designation for ATX-01 in DM1 from the US and European authorities.