FDA fast-tracks review of Merck's new WELIREG (belzutifan) drug submission for advanced kidney cancer patients
Merck, identified as MSD outside the US and Canada, has disclosed that the U.S. FDA has acknowledged and provided priority assessment for an auxiliary new drug application pursuing endorsement for WELIREG. This compound is Merck's oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor, which is intended for the therapy of adult individuals with advanced renal cell carcinoma who have previously received immune checkpoint and anti-angiogenic therapies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The sNDA derives from the findings of the LITESPARK-005 study. The drug WELIREG showed a considerable and clinically relevant enhancement in PFS when compared to everolimus. This information was ascertained from a pre-established interim analysis overseen by an independent Data Monitoring Committee. There was also a notable improvement in the trial's main secondary endpoint, the objective response rate. The FDA assigned a target action date under the Prescription Drug User Fee Act, scheduled for January 17, 2024.
Dr. Marjorie Green, the senior vice president and head of late-stage oncology at Merck Research Laboratories, underscored that “There's a significant unmet need for different treatment options for patients with advanced RCC whose cancer worsens after immunological checkpoint and anti-angiogenic treatments, as they face a worse prognosis."
WELIREG made history as the first approved HIF-2α inhibitor therapy in the U.S., and it's authorized for adult patients with von Hippel-Lindau disease who need treatment for associated RCC, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors when immediate surgery isn't required.
LITESPARK-005 is part of an extensive development plan for WELIREG that includes four Phase 3 trials in RCC. These consist of LITESPARK-011 and LITESPARK-012, which assess WELIREG in second-line and treatment-naïve advanced disease scenarios, along with LITESPARK-022, which examines WELIREG in the adjuvant setting.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
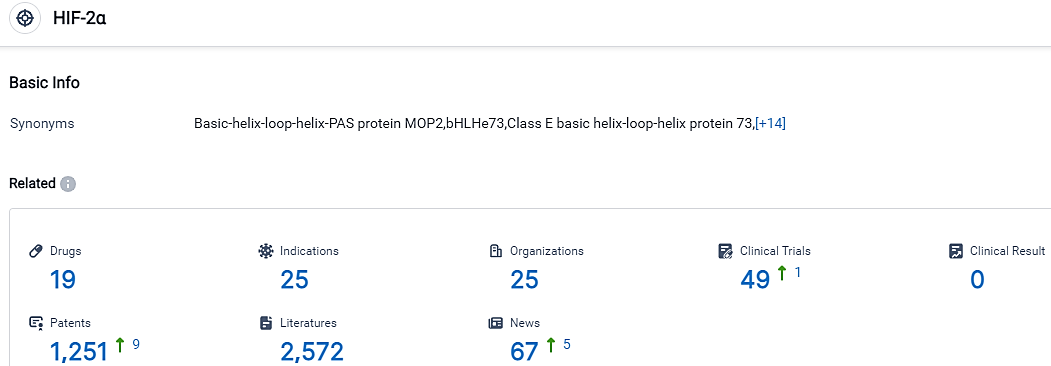
According to the data provided by the Synapse Database, As of September 20, 2023, there are 19 investigational drugs for the HIF-2α target, including 25 indications,25 R&D institutions involved, with related clinical trials reaching 49,and as many as 1251 patents.
Belzutifan targets HIF-2α and has shown promise in treating various diseases across multiple therapeutic areas. With its global approval in 2021 and ongoing phase 3 development in China, Belzutifan has the potential to make a significant impact in the field of biomedicine. Its regulatory designations further emphasize its importance in addressing critical medical needs.