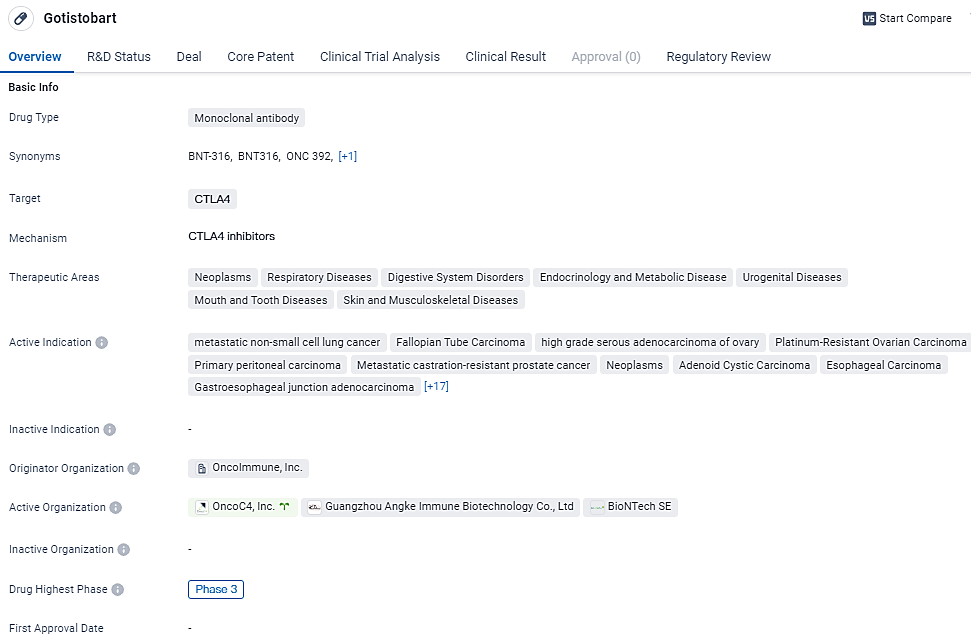
First Late-Stage Prostate Cancer Patient Dosed in OncoC4's BNT316/ONC-392 Study
OncoC4, Inc. has recently disclosed the initiation of treatment for the initial participant in an early to mid-stage clinical study. This participant suffers from prostate cancer that has spread and no longer responds to hormonal therapy designed to lower testosterone. The investigational study is examining the efficacy of the immunotherapy agent BNT316/ONC-392 (gotistobart), which targets CTLA-4. This agent is being tested in conjunction with a radiopharmaceutical treatment known as lutetium vipivotide tetraxetan. The collaborative efforts of BioNTech and OncoC4 are behind the development of BNT316/ONC-392.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Globally, prostate cancer is one of the most frequently identified malignancies in the male population, and it ranks as a leading contributor to cancer-related mortality. An estimated 10-20% of individuals with prostate cancer progress to a stage known as castration-resistant prostate cancer, resulting in a troubling forecast. Median life expectancy for these patients, particularly during the metastatic phase, is roughly two years. The need for innovative therapies that can enhance survival rates for individuals battling metastatic castration-resistant prostate cancer (mCRPC) is still significant.
"Recent advancements in mCRPC treatments have yielded better patient outcomes, yet there is a substantial gap in the availability of gentle targeted treatments that can additionally prolong the lives of these individuals. We are eager to investigate the efficacy of the combination of BNT316/ONC-392 with radioligand therapy, aiming to achieve enhanced results for those facing this progressive disease," commented David R. Wise, M.D., Ph.D., head of the Perlmutter Cancer Center at NYU Langone Health, and the study's lead investigator for the Phase 1/2 clinical trial.
The initial phase of the study will focus on the safety profile of combining BNT316/ONC-392 with lutetium vipivotide tetraxetan to determine an optimal dosage. In its Phase 2 component, the trial will explore both the safety and effectiveness of the combination in patients with metastatic castration-resistant prostate cancer, positive for prostate-specific membrane antigen on scans (PSMA), who have not responded to earlier androgen receptor-targeting treatments and have not yet received lutetium vipivotide tetraxetan. The primary objective of the study is to see if combining these treatments could surpass the prevailing standard in extending progression-free survival for patients.
In March of 2023, BioNTech and OncoC4 declared the commencement of a strategic partnership for the co-development of BNT316/ONC-392 across various solid tumor types, agreeing to share the costs of development equally for these studies. Exclusive international commercial rights for this investigational candidate are held by BioNTech.
At the moment, BNT316/ONC-392's therapeutic potential is under assessment in several active clinical trials focused on advanced solid tumors. This includes the ongoing PRESERVE-003 Phase 3 registrational trial, centered on examining the efficacy of BNT316/ONC-392 as a standalone treatment in patients with metastatic non-small cell lung cancer that has shown resistance to immunotherapeutic interventions.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
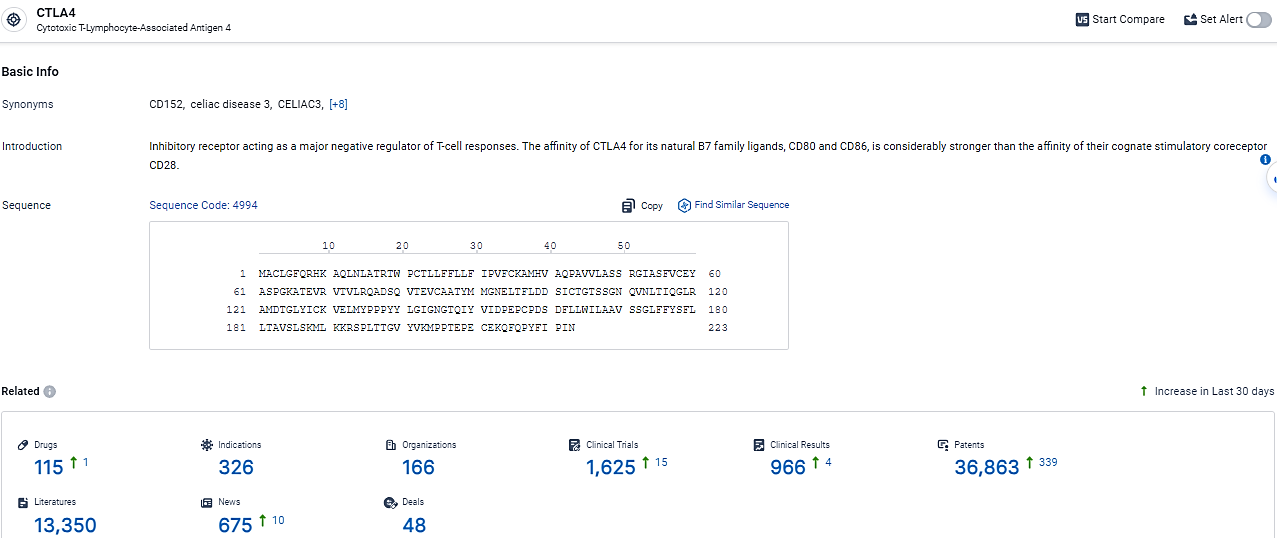 According to the data provided by the Synapse Database, As of February 28, 2024, there are 115 investigational drugs for the CTLA-4 target, including 326 indications, 166 R&D institutions involved, with related clinical trials reaching 1625, and as many as 36863 patents.
According to the data provided by the Synapse Database, As of February 28, 2024, there are 115 investigational drugs for the CTLA-4 target, including 326 indications, 166 R&D institutions involved, with related clinical trials reaching 1625, and as many as 36863 patents.
Gotistobart shows promise as a potential treatment option for a wide range of diseases, particularly various types of cancers. Its monoclonal antibody nature and targeting of CTLA4 make it a unique and potentially effective therapeutic approach. The drug's advancement to Phase 3 trials and regulatory designations further highlight its potential and the recognition of its importance in addressing unmet medical needs.