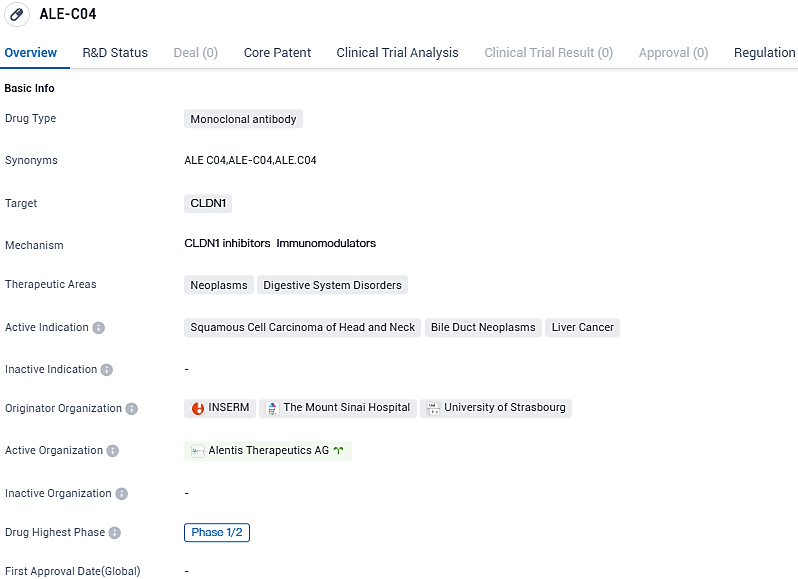
First patient received ALE.C04 treatment in Alentis Therapeutics' Phase 1/2 study for HNSCC
Alentis Therapeutics, an advanced biotech firm focusing on treatments for Claudin-1 positive (CLDN1+) tumors and organ fibrosis, has confirmed that the initial participant has received dosage in a Phase 1/2 clinical study of ALE.C04, an experimental antibody targeting Claudin-1 for handling CLDN1+ tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The individual is part of the Phase I trial, overseen by Dr. Anthony El-Khoueiry, who is the Associate Director of Clinical Research at Norris Comprehensive Cancer Center of the University of Southern California, a branch of the Keck Medicine of USC. "ALE.C04 is an innovative cancer treatment antibody that has exhibited significant potential in preclinical models," stated Dr. Luigi Manenti, Alentis' Chief Medical Officer. "The function of ALE.C04 is to eliminate CLDN1+ tumors directly while overcoming resistance to check-point inhibitor treatment by reinstating immune cell trafficking."
Adding to this, Dr. Jacob Thomas, the primary investigator for the trial at USC Norris and currently an Assistant Professor of Medicine at the Keck School of Medicine of USC, said, "Patients with R/M HNSCC tend to have inadequate outcomes, there is a pressing need for innovative and effective treatments. The action mechanism and the preclinical information on ALE.C04 present a compelling scientific justification for its usage in HNSCC."
Concerning the trial, Prof. Josep Tabernero, leading the Medical Oncology department at Vall d’Hebron University Hospital stated, "This inaugural human trial will generate crucial insights into ALE.C04’s effectiveness as a stand-alone treatment and in conjunction with anti-PD-1 treatment. I am eagerly awaiting the results and the prospect of progressing ALE.C04 for different CLDN1+ tumors, including colorectal cancer.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
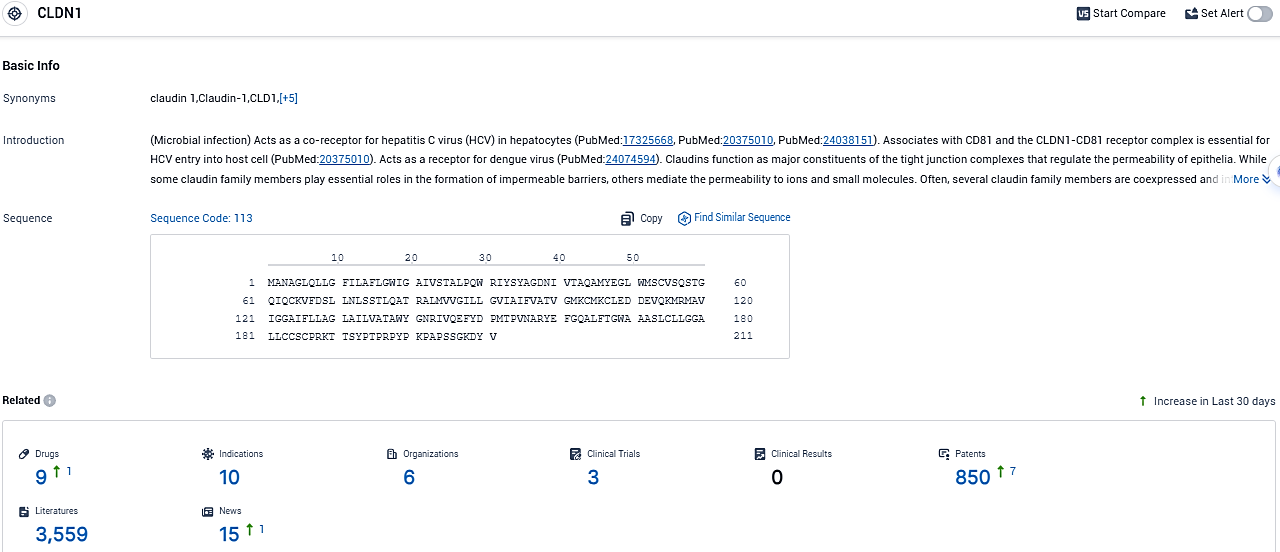
According to the data provided by the Synapse Database, As of November 22, 2023, there are 9 investigational drugs for the CLDN1 target, including 10 indications, 6 R&D institutions involved, with related clinical trials reaching 3, and as many as 850 patents.
ALE.C04 is a first-in-class monoclonal antibody developed to specifically target exposed CLDN1 on cancer cells. This investigational antibody is designed to treat cancer in two ways: remodeling of the extracellular matrix, leading to improved NK and T-cell trafficking and direct tumor cell killing through the effector function. ALE.C04 received Fast Track designation from the Food and Drug Administration for the treatment of CLDN1+ HNSCC.