Fusion Pharma's Early Phase 2 Outcomes for FPI-2265 at AACR 2024
Fusion Pharmaceuticals Inc., an enterprise engaged in clinical-phase cancer research with a commitment to crafting advanced radioconjugates for targeted treatments, has disclosed the preliminary results regarding both the effectiveness and safety aspects from its TATCIST Phase 2 study, an open-label trial concerning FPI-2265. This disclosure took place during the American Association for Cancer Research Annual Meeting 2024, a gathering scheduled from April 5th to April 10th, taking place in San Diego, California.
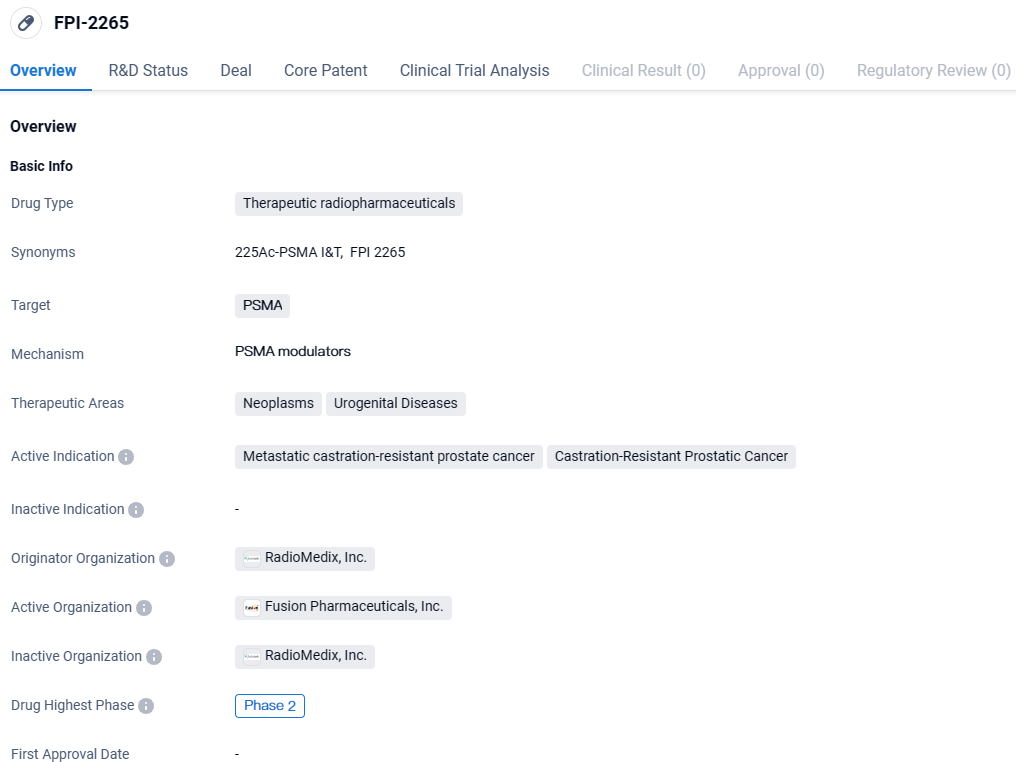
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The study indicates that the investigative drug FPI-2265 exhibits efficacy in individuals with advanced metastatic castrate-resistant prostate cancer who have undergone extensive prior treatments. This includes subjects previously treated with lutetium-involved radioligand therapies. The findings on safety, tolerability, and efficacy align well with existing literature on other 225Ac-PSMA radioligand therapies involving small molecules.
Fusion's CEO, Dr. John Valliant, expressed his views on the promising preliminary findings of the TATCIST study. He emphasized FPI-2265's leading role in the arena of actinium-based PSMA-directed radiopharmaceuticals and its potential to enhance therapeutic strategies for men with mCRPC.
Dr. Valliant added that the early results are indicative of significant clinical benefits and acceptable safety with FPI-2265, reinforcing the pursuit of developing this therapy for patients who have not responded to treatments that include lutetium-based radiopharmaceuticals. He projected the commencement of Phase 2 trials within the framework aimed at regulatory approval in the second quarter of 2024. Considering the growing population of patients transitioning from treatments like PLUVICTO™, FPI-2265 is believed to be a promising candidate to address the pressing healthcare needs of individuals with mCRPC.
The TATCIST study's primary aim is to assess the effectiveness of FPI-2265 in patients with progressive mCRPC. This encompasses both patients who are new to PSMA-focused radioligand therapies and those who have previously received therapies such as 177Lu-based PSMA radioligand treatments, like PLUVICTO™.
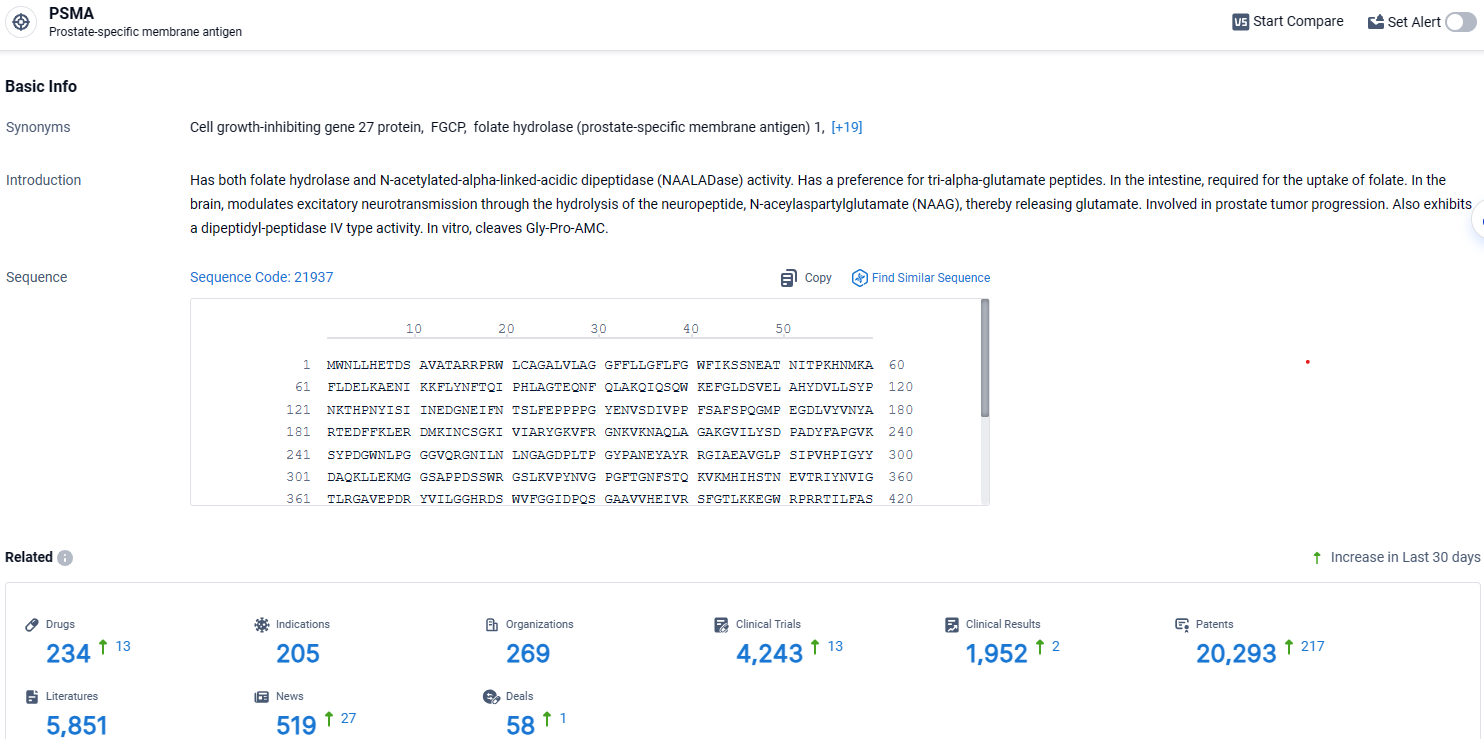
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 11, 2024, there are 234 investigational drugs for the PSMA target, including 205 indications, 269 R&D institutions involved, with related clinical trials reaching 4243, and as many as 20293 patents.
FPI-2265 is an actinium-225 based PSMA targeting RC, for mCRPC, currently in a Phase 2 trial. Actinium-225 emits alpha particles and holds the promise of being a next-generation radioisotope in cancer treatment. By delivering a greater radiation dose over a shorter distance, alpha particles such as actinium-225 have the potential for more potent cancer cell killing, and targeted delivery, thereby minimizing damage to surrounding healthy tissue.