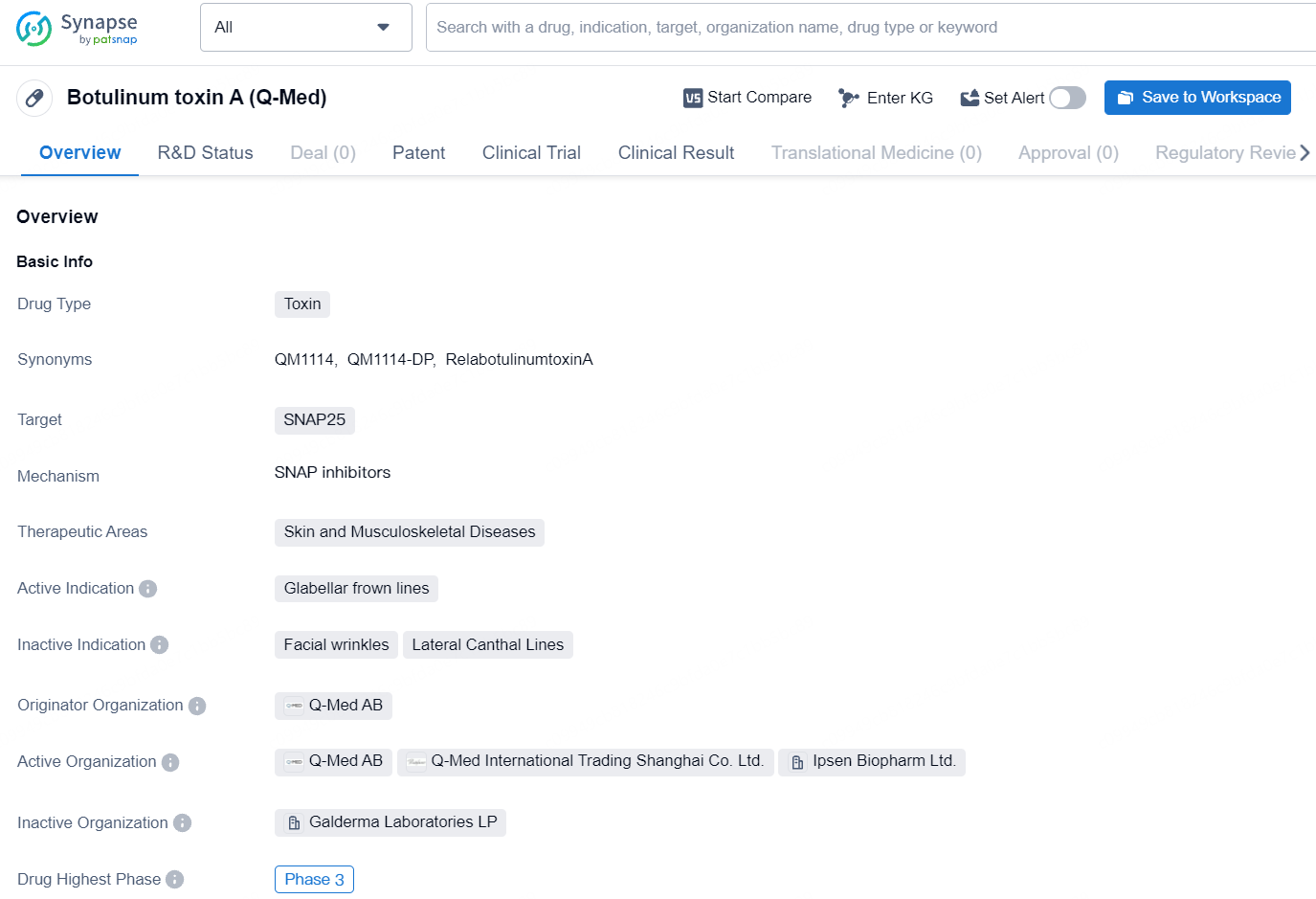
Galderma's Relfydess™ has been approved for use in Europe
Galderma has successfully completed the European decentralized procedure, leading to a favorable decision for RelfydessTM (RelabotulinumtoxinA - previously known as QM1114). RelfydessTM is approved for temporary improvement in the appearance of moderate-to-severe glabellar lines at maximum frown and lateral canthal lines seen at maximum smile, alone or in combination, in adult patients under 65 years old, when the severity of these lines has a significant psychological impact on the patient.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
After completing the DCP successfully, approvals in the 16 relevant countries are currently being finalized. RelfydessTM was also granted marketing authorization in Australia earlier this year.
Manufactured and developed by Galderma, RelfydessTM is the initial ready-to-use liquid neuromodulator made with PEARL™ Technology to maintain molecule integrity.
It delivers a highly active, innovative, and complex-free molecule, with effects seen by up to 39% of patients from the first day and improvements maintained by up to 75% of patients for six months with regard to frown lines and crow’s feet. The product is designed for straightforward volumetric dosing, without requiring reconstitution, to enhance usability and ensure consistent dose/volume.
RelfydessTM, pioneered by Galderma, is the first liquid neuromodulator prepared with PEARLTM Technology to maintain molecule integrity. PEARLTM Technology aims to deliver a highly active, innovative, and complex-free molecule, with initial effects observed by up to 39% of patients and continued improvements seen by up to 75% of patients for six months.
RelfydessTM is tailored for effortless volumetric dosing, avoiding the need for reconstitution, to simplify use and maintain consistent dose/volume for each application. It was exclusively developed and produced by Galderma to broaden its neuromodulator range within the most extensive Injectable Aesthetics portfolio in the market.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
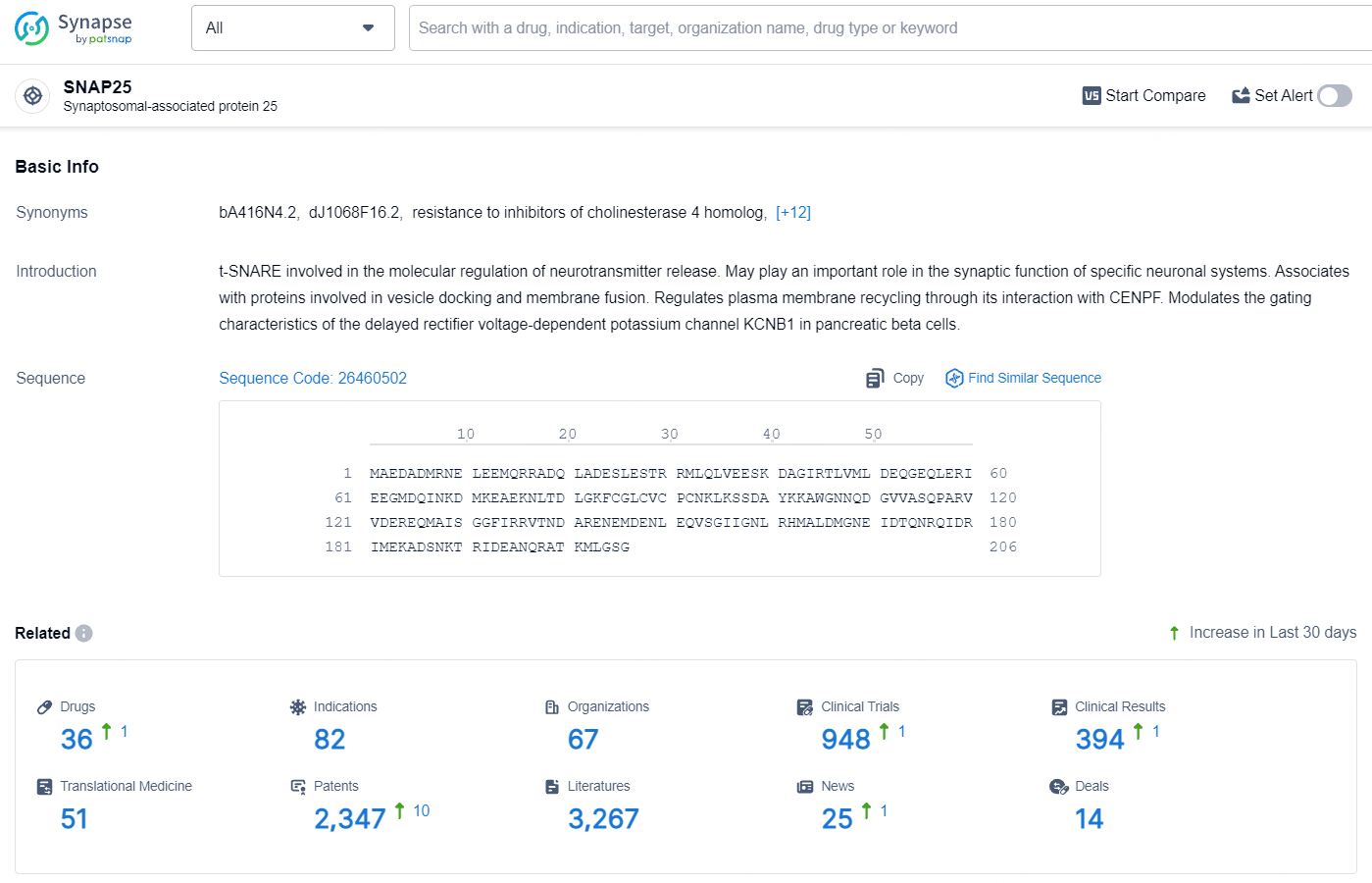
According to the data provided by the Synapse Database, As of August 2, 2024, there are 36 investigational drugs for the SNAP25 target, including 82 indications, 67 R&D institutions involved, with related clinical trials reaching 948, and as many as 2347 patents.
Botulinum toxin A is a toxin-based drug that targets SNAP25 and is primarily used for the treatment of glabellar frown lines in the therapeutic areas of skin and musculoskeletal diseases. Originated by Q-Med AB, the drug has progressed to the highest phase of clinical development, Phase 3, globally and in China, indicating its potential for future commercialization and availability to patients in need.