Gilead Shares Latest Information on the Phase 3 TROPiCS-04 Trial
Gilead Sciences, Inc. disclosed primary outcomes from the Phase 3 TROPiCS-04 verification study involving patients with locally advanced or metastatic urothelial cancer. This TROPiCS-04 trial compared the effects of Trodelvy (sacituzumab govitecan-hziy; SG) against single-agent chemotherapy in individuals with metastatic urothelial carcinoma (mUC) who had prior treatments with platinum-based chemotherapy and anti-PD-(L)1 therapy.
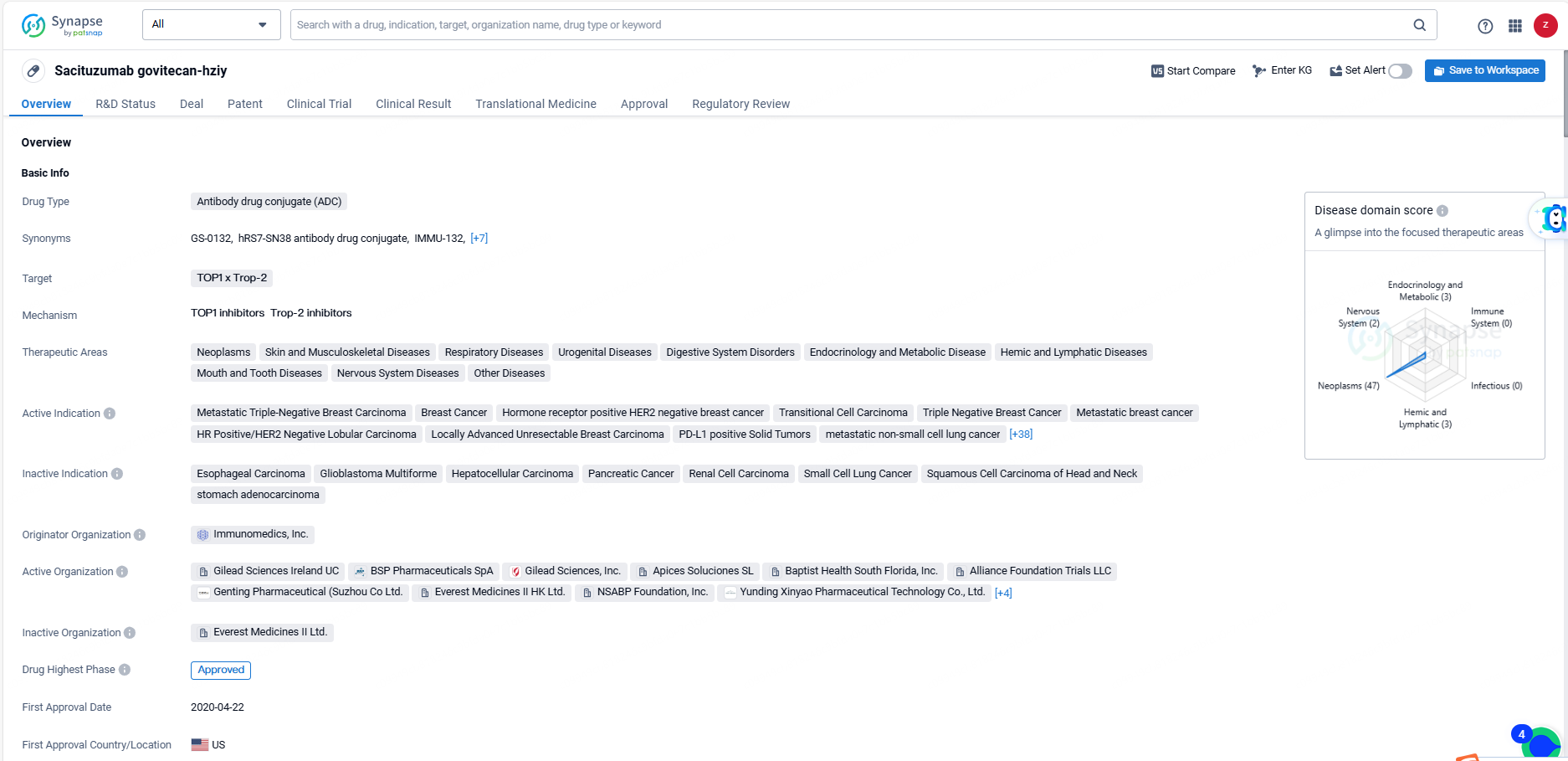
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The trial did not achieve its primary goal of overall survival in the intention-to-treat group. Nonetheless, Trodelvy showed a numerical benefit in OS, with favorable trends in select pre-specified subgroups and secondary endpoints such as progression-free survival and overall response rate. These subgroup analyses were not subjected to alpha control for formal statistical assessment. These findings will be shared at an upcoming medical conference.
In the entire study cohort, there was a higher incidence of deaths attributable to adverse events associated with Trodelvy compared to TPC. These were primarily early in the treatment phase and were related to neutropenic complications, including infections. Gilead will be conducting further investigations into these findings and is emphasizing to healthcare providers the critical importance of using granulocyte-colony stimulating factor to prevent neutropenic complications. Trodelvy carries a Boxed Warning for severe or life-threatening neutropenia; detailed Safety Information is provided below.
The established safety profile of Trodelvy for its approved breast cancer indications and other investigational uses remains unchanged. So far, the safety profile of Trodelvy has been generally well-tolerated and consistent with its use in over 40,000 patients across approved indications and clinical trials.
Gilead continues to review the data and will discuss the outcomes and future actions with the FDA. In the United States, Trodelvy holds an accelerated approval for patients with locally advanced or metastatic urothelial cancer who have undergone prior treatment with platinum-based chemotherapy and anti-PD-(L)1 therapy. This approval is based on tumor response rate and duration of response. Ongoing approval for this indication may depend on the confirmation and description of clinical benefits in confirmatory trials, such as the TROPiCS-04 study.
Trodelvy (sacituzumab govitecan-hziy) is a first-in-class antibody-drug conjugate targeting Trop-2. Trop-2 is a cell surface antigen highly present in various tumor types, including over 90% of breast, bladder, and lung cancers. Trodelvy is designed with a proprietary hydrolyzable linker connected to SN-38, a topoisomerase I inhibitor payload. This unique configuration delivers strong activity to both Trop-2 expressing cells and the tumor microenvironment through a bystander effect.
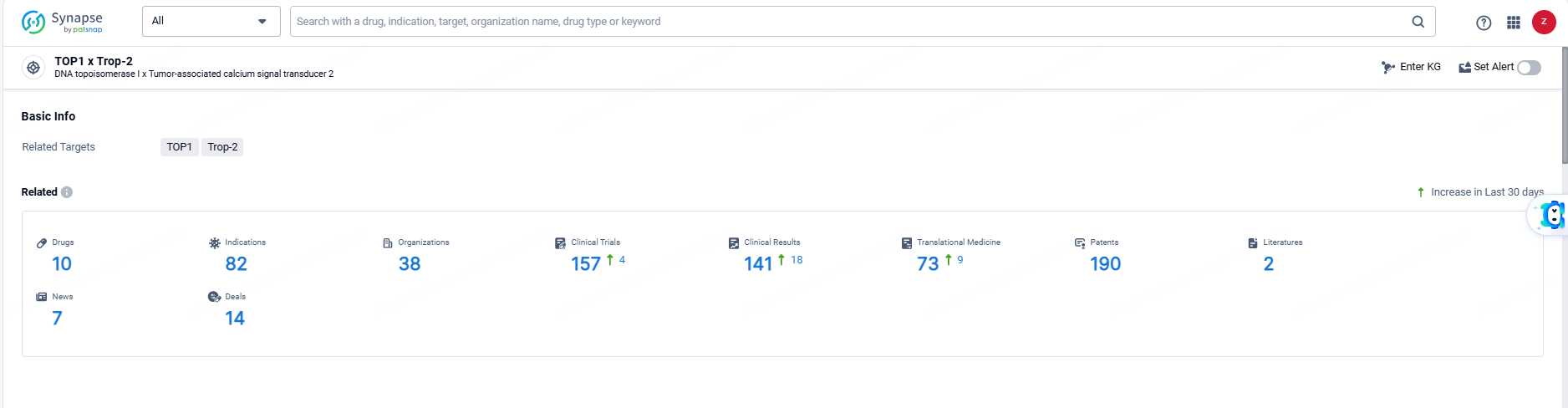
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 6, 2024, there are 10 investigational drugs for the Trop-2 and TOP1 targets, including 82 indications, 38 R&D institutions involved, with related clinical trials reaching 157, and as many as 190 patents.
Trodelvy was developed by Immunomedics, Inc. and has received approval in the United States in April 2020. It has been granted various regulatory designations including priority review, accelerated approval, fast track, orphan drug, and breakthrough therapy. The highest phase of approval for Sacituzumab govitecan-hziy is approved both globally and in China. This information provides a comprehensive overview of the drug's indications, regulatory status, and originator organization, without any subjective interpretations.