GV20 Therapeutics Announces Promising GV20-0251 Phase 1 Results at 2024 ESMO Congress
GV20 Therapeutics, an advanced biotherapeutics firm leveraging AI, has disclosed the outcomes of its clinical trial Phase 1/2 for GV20-0251 at the ESMO Congress 2024 held in Barcelona, Spain.
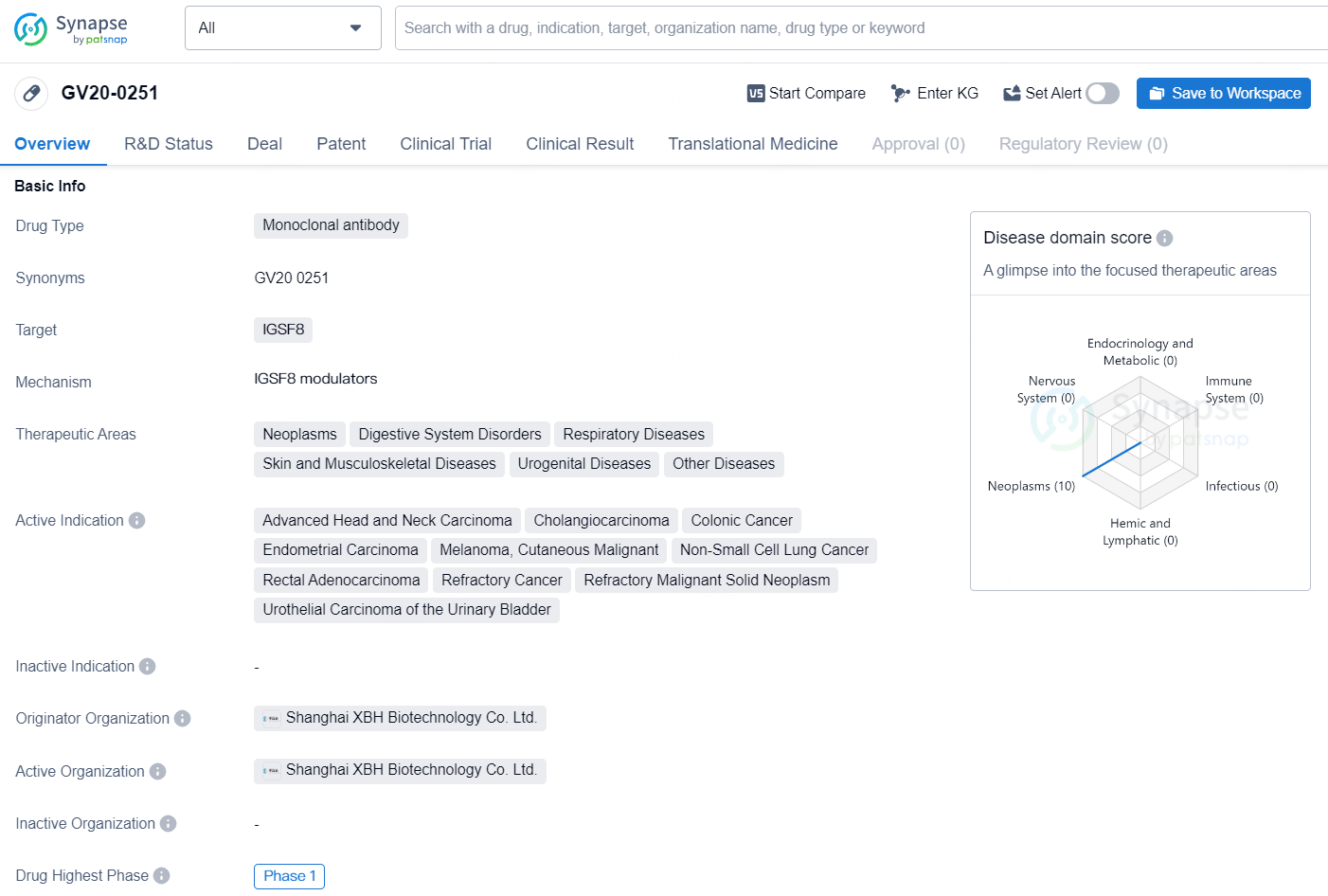
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Kristopher Wentzel from The Angeles Clinic and Research Institute delivered a presentation discussing the clinical data from the monotherapy dose escalation segment of the ongoing investigation of GV20-0251, an innovative antibody targeting the immune checkpoint IGSF8, in patients with advanced solid tumors (NCT05669430).
Key points from the presentation were:
Patient Demographics: The study included 38 heavily pre-treated patients over six dose levels and two dosing schedules. The median age was 62 years, and patients had a median of 4 prior lines of treatment.
Safety Profile: GV20-0251 was generally well-tolerated at all dose levels (ranging from 0.5 to 20 mg/kg), with no dose-limiting toxicities noted. The majority of treatment-related adverse events were grade 1/2, with only one grade 3 case of pneumonitis reported.
Efficacy Results: In 12 efficacy-evaluable metastatic cutaneous melanoma patients, two confirmed partial responses were noted. Additionally, stable disease was observed in 14 of the 29 efficacy-evaluable patients, including 4 who experienced tumor shrinkage.
Pharmacokinetics: The pharmacokinetics were dose-proportional with a half-life around 25.6 days. Full target occupancy on circulating T cells was observed at doses of 3 mg/kg and above.
“We are buoyed by these positive findings for GV20-0251, which marks a pivotal achievement as the first clinical data for an AI-engineered antibody aimed at an AI-identified target,” remarked Dr. Shirley Liu, Co-founder and Chief Executive Officer of GV20 Therapeutics. “The favorable safety profile and early signs of efficacy, especially in melanoma patients, underscore the potential of IGSF8 as an innovative immune checkpoint target.”
GV20-0251 has been developed to boost NK cell-mediated killing of malignant cells, enhance dendritic cell antigen presentation, and elevate T cell signaling. This approach could be notably beneficial for patients with tumors that are deficient in antigen presentation, which typically show resistance to current immune checkpoint inhibitors.
The company is actively enrolling cancer patients who have relapsed on or are refractory to anti-PD1 therapies. These patients will be treated with GV20-0251 in combination with pembrolizumab to further assess the drug’s safety, pharmacokinetics, pharmacodynamics, and efficacy.
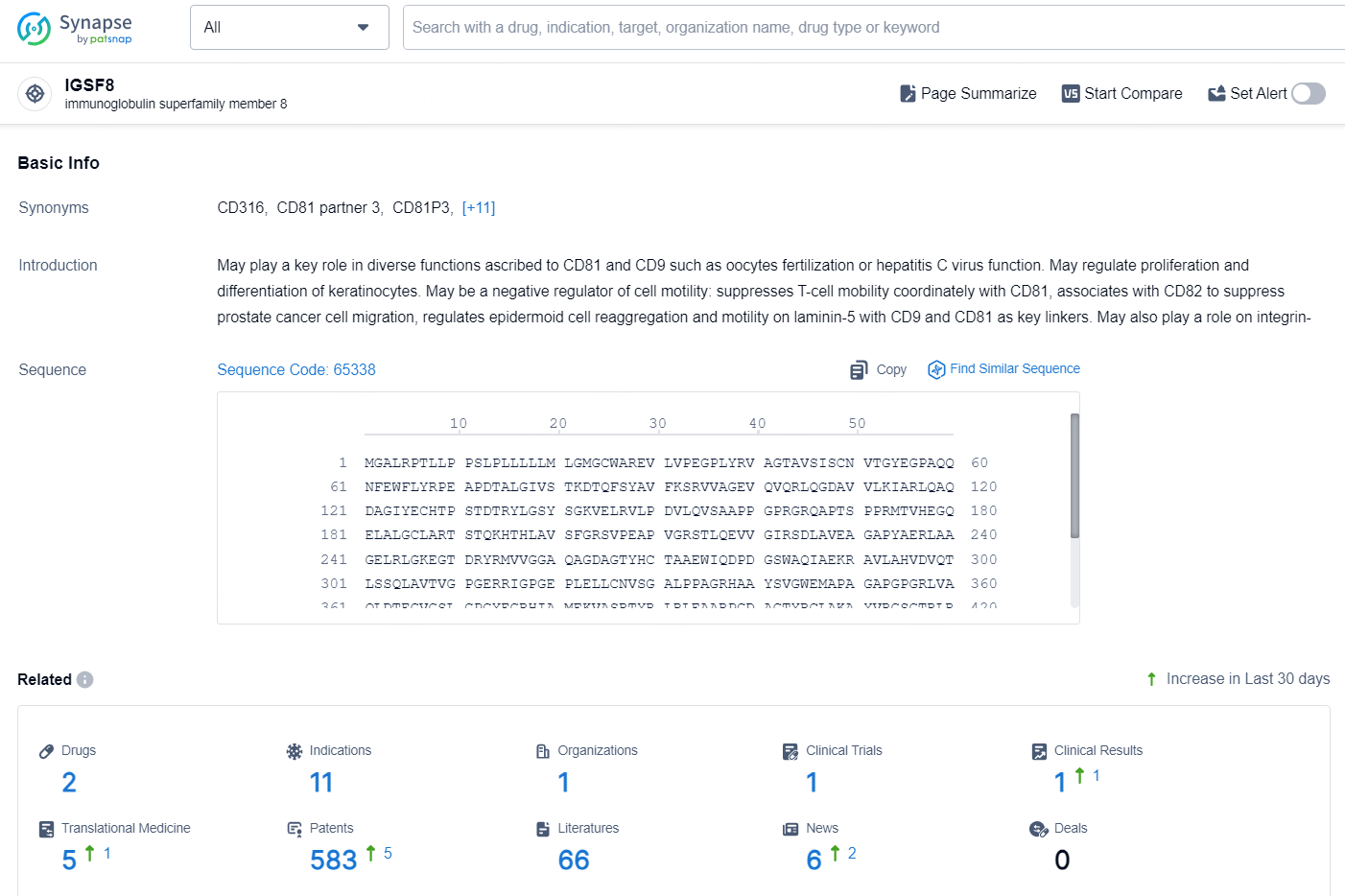
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 2 investigational drugs for the IGSF8 targets, including 11 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 583 patents.
The drug GV20-0251 is a monoclonal antibody that targets the IGSF8 protein. It is being developed for the treatment of a wide range of therapeutic areas, including Neoplasms, Digestive System Disorders, Respiratory Diseases, Skin and Musculoskeletal Diseases, Urogenital Diseases, and Other Diseases. The drug is currently indicated for the treatment of Advanced Head and Neck Carcinoma, Cholangiocarcinoma, Colonic Cancer, Endometrial Carcinoma, Melanoma, Cutaneous Malignant, Non-Small Cell Lung Cancer, Rectal Adenocarcinoma, Refractory Cancer, Refractory Malignant Solid Neoplasm, and Urothelial Carcinoma of the Urinary Bladder.