Halia Therapeutics began an early-stage Phase 2a trial for HT-6184, a new NLRP3 inhibitor, treating the first patient for less severe myelodysplastic conditions
Halia Therapeutics, an enterprise at the forefront of developing innovative small molecule therapeutic agents to tackle inflammation, has declared that the initial participant has been administered a dose in the stage 2a clinical study. This trial is focused on appraising the effectiveness of their primary drug, HT-6184, which is being tested as a potential treatment for myelodysplastic syndromes categorized as lower-risk.
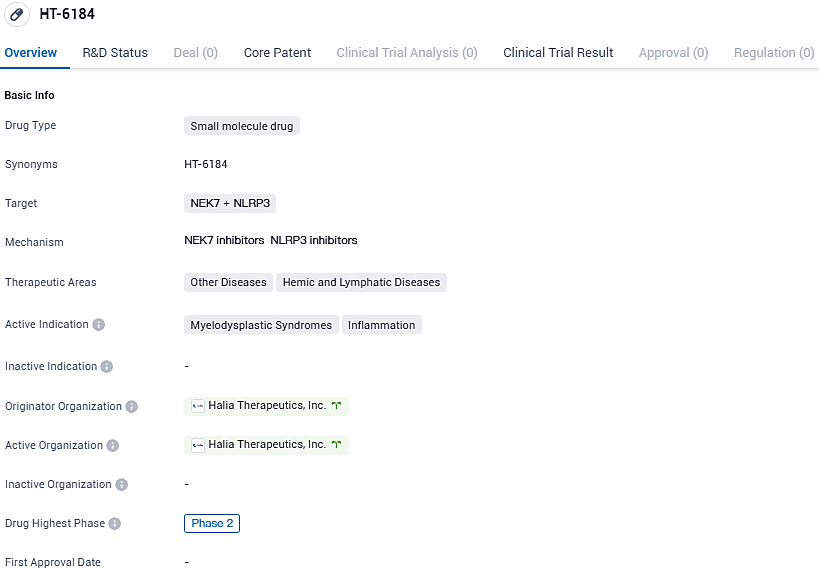
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
HT-6184 represents an innovative and orally administered pioneer molecule targeting the NLRP3/NEK7 inflammasome pathway, known for its central role in propagating inflammatory disorders, along with blood cancers and various other malignancies.
Myelodysplastic syndromes constitute a collection of hematological malignancies characterized by the production of poorly formed cells within the bone marrow, discernible by their irregular size, shape, or structure, often referred to as “dysplastic.” This condition leads to a shortage of functional blood cells, which may give rise to several health issues, including, but not limited to, anemia, frequent infections, and potential progression to acute cancer forms. The clinical study will assess the efficacy and tolerability of HT-6184 in a cohort of up to 40 patients diagnosed with LR-MDS, with research activities spread out over various locations in India.
The research aims to quantify the improvement in blood cell production, with a sharp focus on reduction in the need for blood transfusions and variations in hemoglobin concentration, which have been designated as the primary outcomes. Additionally, secondary outcomes will scrutinize the influence of HT-6184 on indicators showcasing the activity of the inflammasome within MDS, as well as shifts in the prevalence of clonal mutations in specific genes. Projected completion of this study is slated for the fourth quarter of 2025.
"The commencement of HT-6184's Phase 2 clinical trial, with the first patient receiving the dose, signifies a pivotal development for our company, Halia Therapeutics, in our endeavor to examine the effects of NLRP3 inflammasome inhibition in managing a host of immune and inflammatory conditions," announced Margit M. Janát-Amsbury, MD, Ph.D., the Medical Chief at Halia Therapeutics.
Dr. Janát-Amsbury added, "The affirming findings from our initial Phase I trial investigating HT-6184 have brought to light the compound's capacity to diminish pro-inflammatory cytokines. With the initiation of this advanced phase of clinical exploration, our team is optimistic about discovering the therapeutic potential of our inflammasome-targeting molecule for individuals with MDS, alongside patients contending with diseases linked to chronic inflammation."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
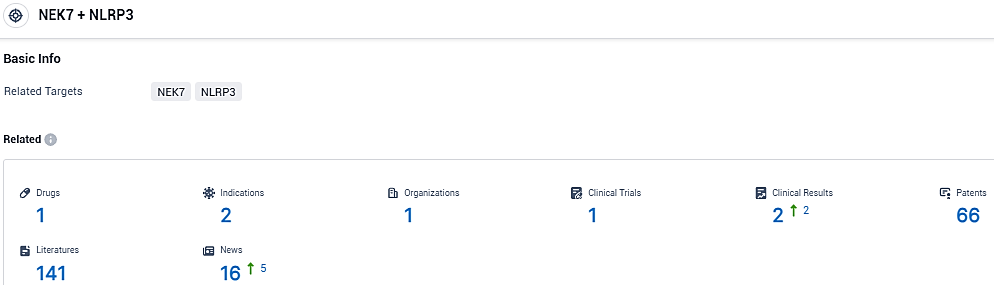
According to the data provided by the Synapse Database, As of December 23, 2023, there are 1 investigational drugs for the NLRP3/NEK7 target, including 2 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 66 patents.
HT-6184 represents an innovative approach as it is the first drug candidate to target the protein NEK7 through an allosteric mechanism. NEK7 is an essential component of the NLRP3 inflammasome and is critical for its assembly and the maintenance of NLRP3 activity. In preclinical models, Halia has shown that inhibiting the ability of NEK7 to bind to NLRP3 leads to a disruption in the formation of the NLRP3 inflammasome complex, thereby inhibiting the signaling from the inflammasome and reducing the inflammatory response.