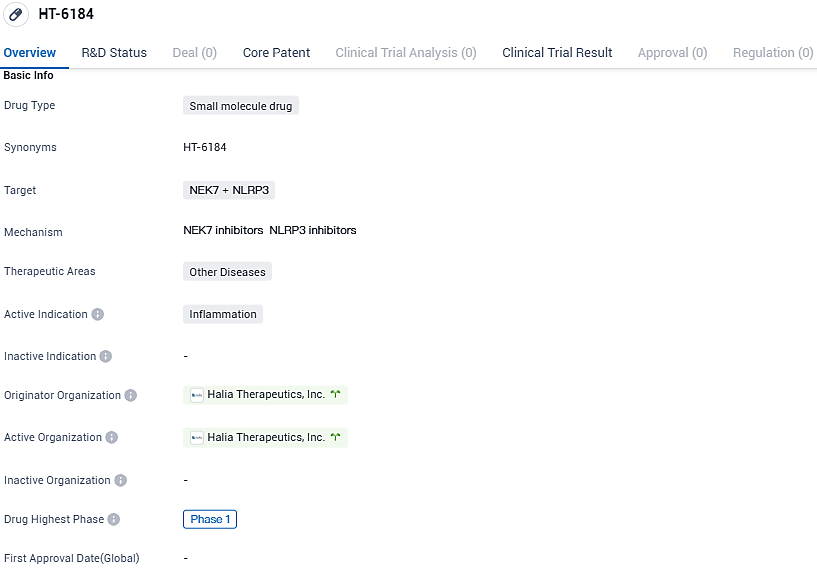
Halia Therapeutics Unveils Positive Early Results for HT-6184 Drug at 5th Inflammasome Summit
Halia Therapeutics, an innovative biotech firm engaged in the early phases of drug development, recently unveiled encouraging outcomes after finishing a Phase 1 clinical study of their premier drug, HT-6184. This drug is at the forefront of a new generation of small molecule drugs, uniquely created to target and reduce inflammation. HT-6184 has been identified as a specific and orally absorbable suppressor of the NLRP3/NEK7 pathway, which is a critical factor in the progression of diseases related to immune inflammation, blood cancers, and potentially other forms of cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The optimism from Halia Therapeutics is high given the favorable findings of the research regarding their product, HT-6184, which serves as an inhibitor of the inflammasome. The company's top executive, Dr. Dave J. Bearss, has confirmed the substance's generally safe profile and its efficacy in diminishing inflammatory cytokines connected to the NLRP3 inflammasome.
"Disorders linked to long-standing inflammation due to the improper activation of inflammasomes have been known for a while, and it's heartening to see that HT-6184 could potentially prevent this inflammation by interfering with the construction of the inflammasome," asserted Dr. Bearss, reinforcing the grounds for optimism and suggesting that these initial results warrant additional clinical exploration to further affirm HT-6184's benefits.
The initial human trial of HT-6184, identified as NCT05447546, was conducted in a controlled, blinded manner with a group of healthy individuals at a single site. This phase included sequential, increasing doses to evaluate its safety and effects, alongside the study of how the body processes and responds to the drug.
It is recognized that the stimulation of NLRP3 instigates the secretion of inflammatory-mediating proteins such as IL-1β and IL-18 and can cause a destructive form of cell death known as pyroptosis. The continuous overactivity of the NLRP3 inflammasome is hypothesized to be a fundamental element leading to the development and worsening of various chronic conditions, including those affecting connective tissue, skin, and the body's immune system reflexes. Moreover, the role of such activation is substantial in grave neurological disorders, with Alzheimer's disease, Parkinson's disease, and multiple sclerosis among those significantly affected by NLRP3.
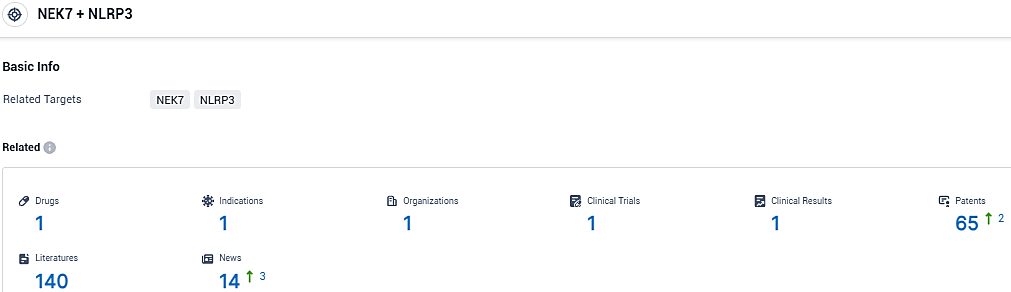
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 7, 2023, there are 1 investigational drugs for the NLRP3/NEK7 target, including 1 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 65 patents.
HT-6184 represents an innovative approach as it is the first drug candidate to target the protein NEK7 through an allosteric mechanism. Preclinical models also showed that in addition to disrupting the formation of the NLRP3 inflammasome, HT-6184 promotes the disassembly of the inflammasome once activated.