Henlius and Intas Receive CHMP Endorsement for HETRONIFLY® as First-Line Treatment for Extensive-Stage Small Cell Lung Cancer in Adults in Europe
Intas Pharmaceuticals Limited ("Intas") has disclosed that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has rendered a favorable opinion, advising the sanctioning of HETRONIFLY® (serplulimab, known as Hansizhuang in China), for distribution in European markets.
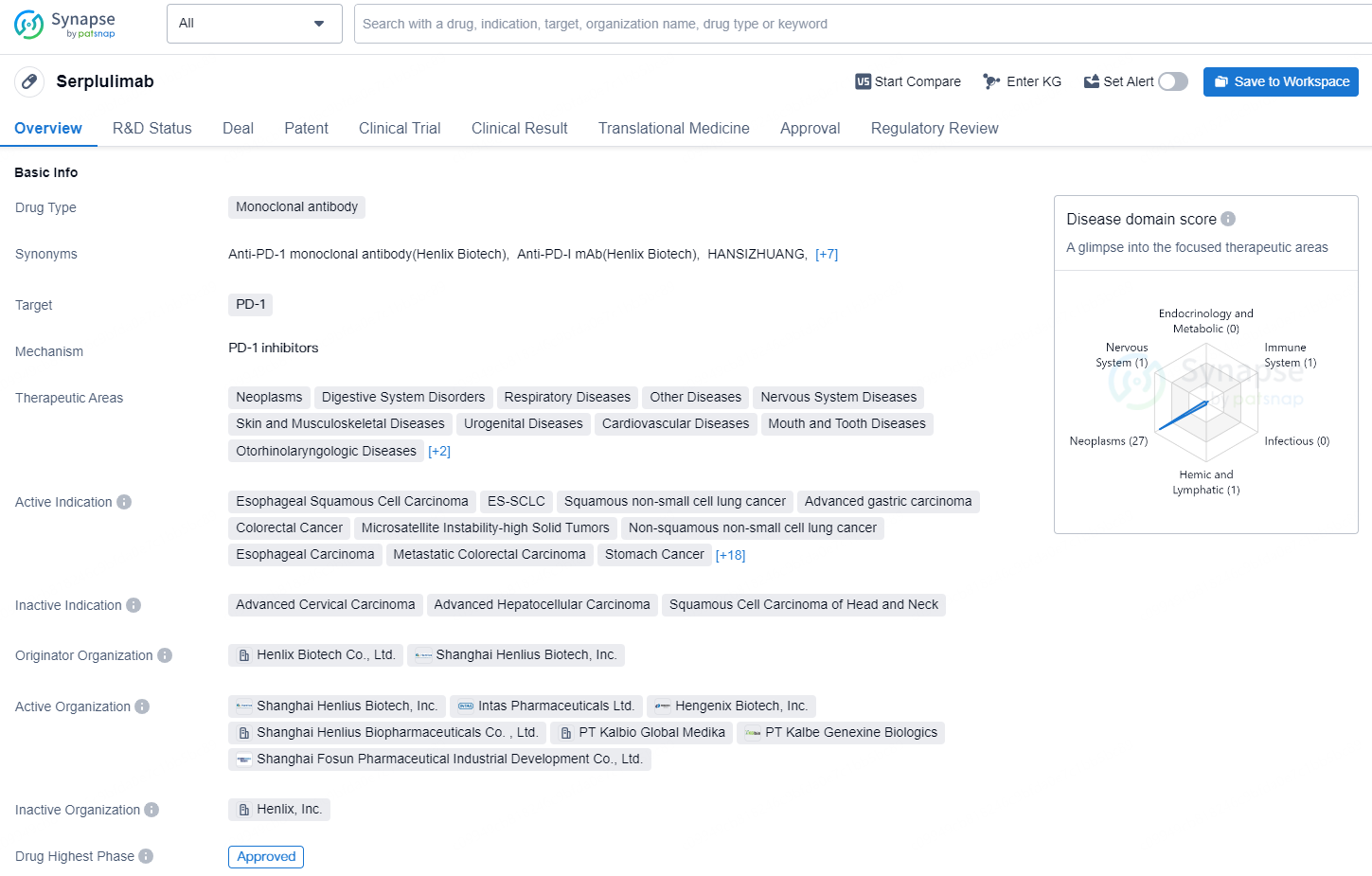
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Serplulimab, a recombinant humanised anti-PD-1 monoclonal antibody (mAb) injection, is Henlius' pioneering monoclonal antibody creation. It has achieved orphan drug status from both the U.S. Food and Drug Administration (FDA) and the European Commission (EC) for the treatment of Small Cell Lung Cancer (SCLC).
Intas, via its subsidiary Accord Healthcare Ltd (Accord), will handle the commercialization of Serplulimab in over 30 European countries. With a significant presence in the global oncology market, Accord is dedicated to oncology and has proven commercial expertise, currently distributing nearly one-third of injectable oncology medications in Europe. This favorable CHMP opinion brings Henlius and Intas closer to making Serplulimab available to European patients.
Dr. Jason Zhu, Executive Director and CEO of Henlius, remarked, “The positive CHMP opinion is a critical milestone in advancing our products globally, further endorsing Henlius’ patient-focused R&D and global strategy. We anticipate formal approval in Europe, which will provide more treatment options and hope to patients both in Europe and globally.”
Paul Tredwell, Executive VP of EMENA at Accord, expressed, "I am delighted with the CHMP’s positive opinion. It not only bolsters our collaboration with Henlius but also indicates that Serplulimab is on its way to becoming a treatment option for patients with extensive stage small cell lung cancer, who currently have limited choices and poor prognosis.”
Alex Falgas, Senior VP of Business Development at Accord, commented, “The CHMP’s positive opinion on Serplulimab is a significant milestone in our effort to deliver top-tier cancer treatments to European patients. This enhances our oncology portfolio and solidifies Accord’s commitment to reducing the global cancer burden by providing innovative therapies to those in need.”
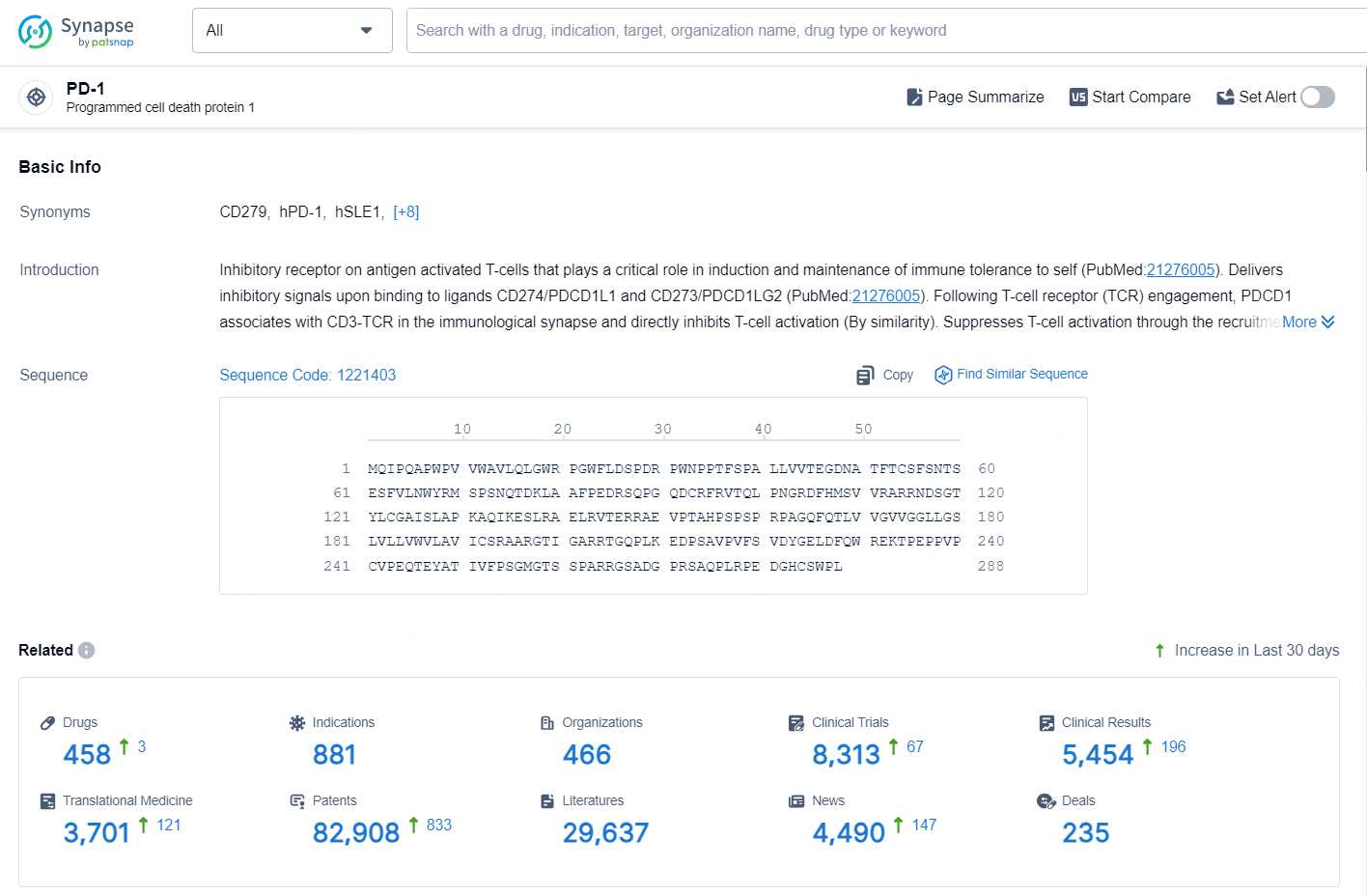
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 24, 2024, there are 458 investigational drugs for the PD-1 targets, including 881 indications, 466 R&D institutions involved, with related clinical trials reaching 8313, and as many as 82908 patents.
Serplulimab is a monoclonal antibody drug that targets PD-1 and has been approved for use in China. The drug has a broad range of therapeutic areas, including neoplasms, digestive system disorders, respiratory diseases, nervous system diseases, and immune system diseases, among others. Serplulimab is indicated for treating various types of cancers, such as esophageal squamous cell carcinoma, non-small cell lung cancer, stomach cancer, colorectal cancer, and hepatocellular carcinoma, among others. It is also used in the treatment of diseases related to the skin, musculoskeletal system, urogenital system, cardiovascular system, and mouth and teeth.