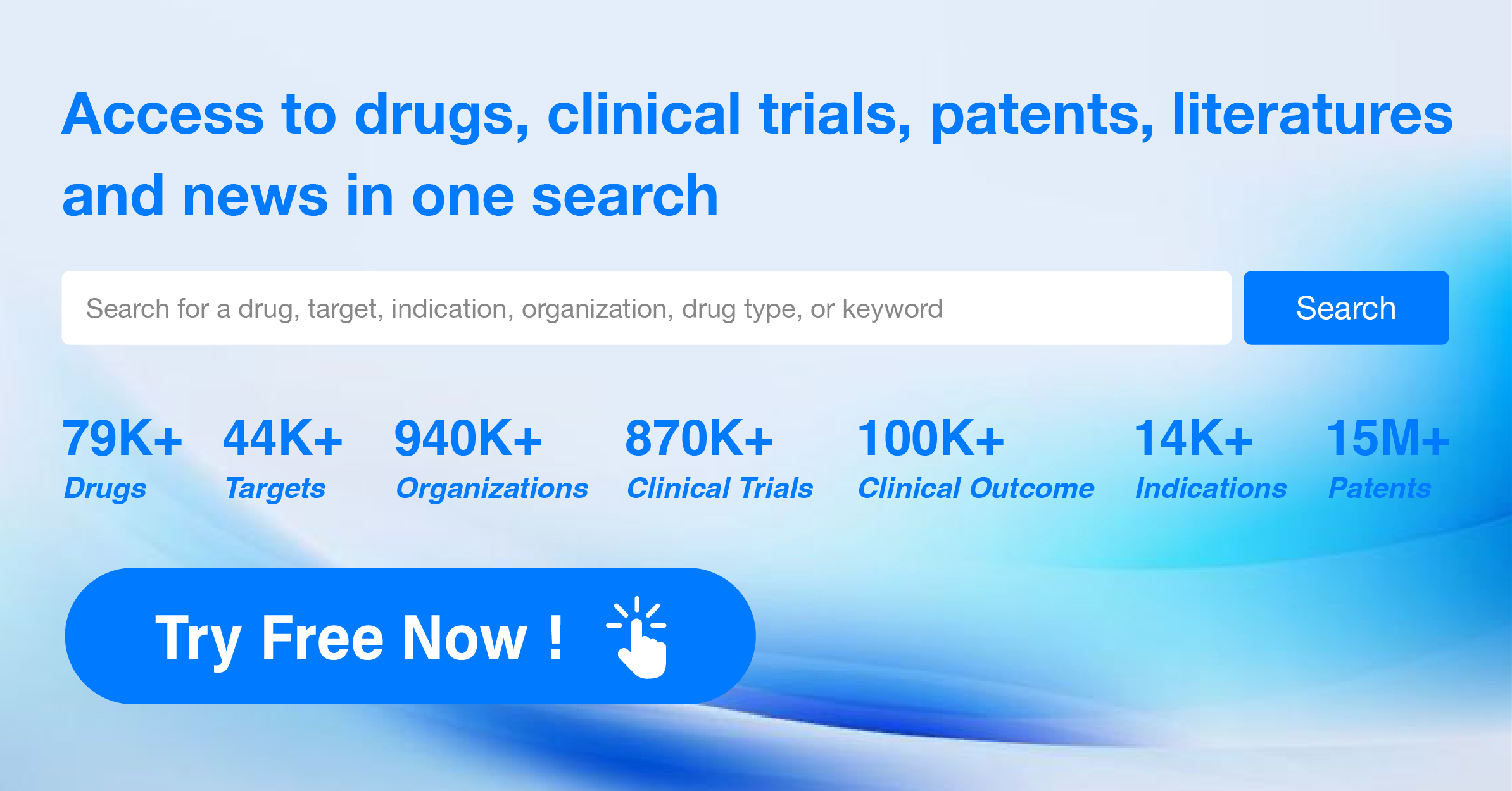
How many sequence modifications exist in the protein sequence?
It is hard to determine how many types of protein sequence modifications exist due to post-translational modifications (PTMs), a natural process where additional components are added or removed from the protein sequence after it is initially generated. These modifications, which can alter the function, cellular localization, and lifespan of proteins, include:
1. Phosphorylation: The addition of a phosphate group, usually to serine, threonine, or tyrosine residues.
2. Acetylation: One of the most common modifications, typically occurring at the N-terminus of the protein or at lysine residues.
3. Ubiquitination: A stepwise process involving the attachment of ubiquitin, a small regulatory protein, to lysine residues. Ubiquitination often marks proteins for degradation.
4. Methylation: Addition of a methyl group, commonly to lysine or arginine residues, leading to structural changes in proteins.
5. Glycosylation: Covalent attachment of sugar molecules, resulting in O-linked (usually attached to serine or threonine) or N-linked (attached to asparagine) glycosylation.
6. Sumoylation: Similar to ubiquitination, a small ubiquitin-like modifier (SUMO) protein is attached to certain lysine residues.
7. Sulfation: Addition of a sulfate group, frequently on tyrosine residues.
8. Prenylation: The addition of prenyl groups to certain cysteine residues, often involved in membrane-targeting.
9. Carboxylation: The addition of a carboxyl group, often to glutamate residues.
10. Nitrosylation: The addition of a nitrosyl group, typically to cysteine residues.
The exact number of protein modifications is difficult to estimate due to the complexity and variability of these processes, but there are at least 200 types of known PTMs. The specific proteins and residues they modify, their timing, and their functional effects can all vary.